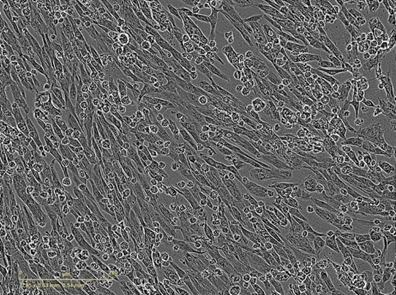
CELLvo™ Human Induced Pluripotent Stem Cell-Derived Atrial Cardiomyocytes are chamber-specific, somatic cells derived from human pluripotent stem cells generated using non-integrating vectors.
Key Features
Chamber Specific Atrial Myocytes. The use of cardiomyocytes that are non-chamber specific and dominated by a ventricular phenotype makes it difficult to study drugs and conditions in vitro that can cause AFib and not VFib.
Non-Gene Edited. Other commercially available cardiomyocytes have a genetically encoded kill switch to eliminate non-myocytes. Unlike CELLvo™ Atrial Cardiomyocytes, many of the iPSCs used for starting material are produced with integrating vectors.
Purified using MACS. This purification technique is used in products that are in the clinic today. The dominant method for purifying cardiomyocytes is glucose starvation which can leave the cells with an injured phenotype.
Differentiated using Small Molecules. Stronger differentiation methods have been developed but are likely to leave behind transcriptomic footprinting.
7-Day Maturation. CELLvo™ Atrial Cardiomyocytes have been validated to mature in as little as 7 days when cultured on CELLvo™ Matrix Plus.
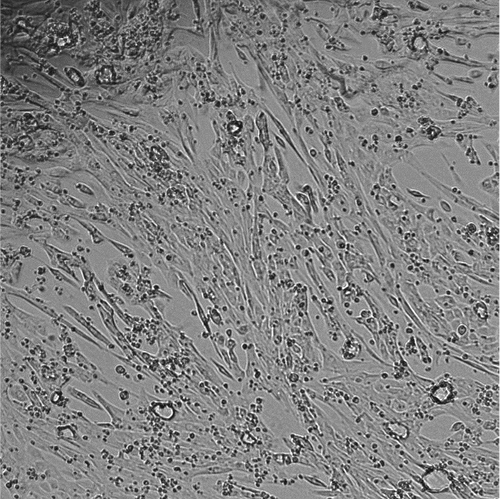
CELLvo™ Matrix Plus Promotes Stem Cell-Derived Cardiomyocyte Maturation
Cardiomyocyte maturation is a major obstacle to the use of iPSC-derived cardiomyocytes for basic research as well as pre-clinical drug and toxicity screening. StemBioSys CELLvo™ Human Atrial iPS-Cardiomyocytes seeded onto CELLvo™ Matrix Plus rapidly and spontaneously align and become structurally mature. Many groups are using electrical pacing, 3-D microtissues with mechanical stimulation, or very long culture periods to achieve some degree of maturation. CELLvo™ Matrix Plus is an easy-to-use solution for cardiomyocyte maturation in 2D culture.
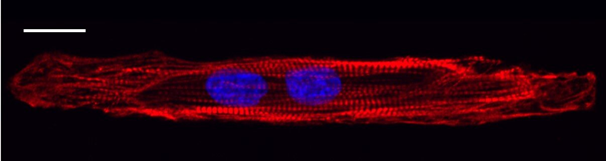
Within 7 days of seeding on CELLvo™ Matrix Plus, a large percentage of commercially available iPSC-derived cardiomyocytes become rod-shaped and bi-nucleated. These are markers of maturation seldom seen using traditional methods. In addition, cardiomyocytes form a uniform, structurally mature, and highly organised monolayer after 7 days in culture.
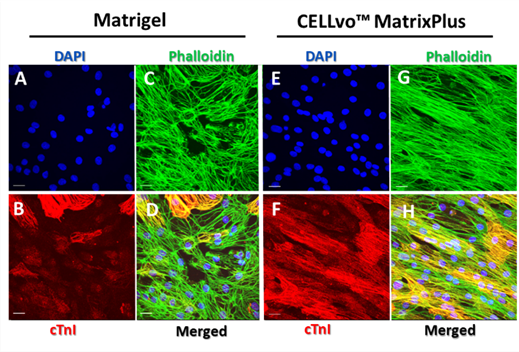
Cardiomyocytes on CELLvo™ Matrix Plus also express uniformly high levels of cardiac troponin I (cTnI), relative to Matrigel. This is especially interesting as this gene is not expressed prenatally.
A voltage-sensitive dye that fluoresces when cells depolarise is used to visualise action potential propagation. Cells on Matrigel have an extended action potential relative to the control condition (not shown). When seeded on CELLvo™ Matrix Plus, cells mature, and an “arrhythmia-in-a-dish” can be visualised. Re-entrant activation results in stable rotor formation resembling to a TdP arrhythmia in vivo.
Shown above are videos of iPSC-CM monolayers in the presence of a cardiotoxic compound.
Publications:
- In work led by Dr. Todd Herron of the University of Michigan Cardiovascular Regeneration Core Laboratory, the value of using mature chamber-specific cardiomyocytes is clearly demonstrated. These cells have structure, metabolism, and electrophysiology that are distinct from what is typically used in in vitro studies. Importantly, these cells are distinct in ways that more closely resemble the reality of cells in the body.
- Recently published in Nature Scientific Reports, hiPSC-CMs expanded on CELLvo™ Matrix Plus were shown to promote efficient development of highly functional, biologically relevant human cardiomyocytes useful for predicting cardiac safety of drugs in early development. Todd Herron, PhD, one of the founders of Cartox, and Travis Block, PhD, Chief Technology Officer of StemBioSys collaborated on the research.
VIDEO: Dr. Todd Herron and Dr. Travis Block discuss hiPSC-CMs expanded on CELLvo™ Matrix Plus
Todd Herron, Ph.D.*, a published expert and one of the founders of Cartox, and Travis Block, Ph.D., Chief Technology Officer of StemBioSys, discuss their recent collaborative research. Highlights of the experiment were reviewed, including successfull maturation of hiPSC-CM that allows relevant testing to predict the cardiac safety of drugs in the early development stage.
Caltag Medsystems is the distributor of StemBioSys products in the UK and Ireland. If you have any questions about these products, please contact us.