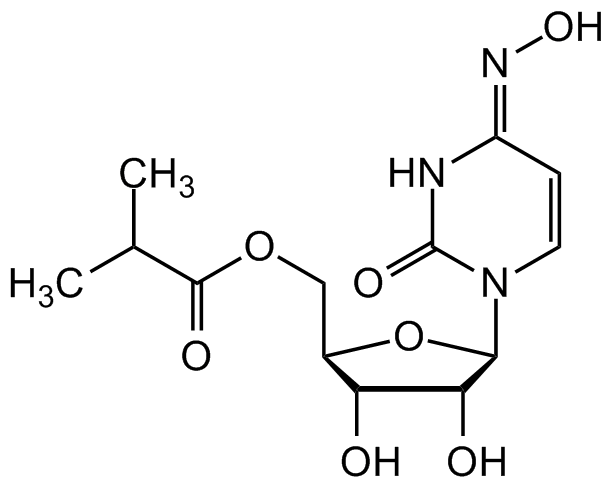
EIDD-2801
| Code | Size | Price |
|---|
| AG-CR1-3733-M010 | 10 mg | £60.00 |
Quantity:
| AG-CR1-3733-M050 | 50 mg | £140.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Images
Documents
Further Information
Alternate Names/Synonyms:
beta-D-N4-Hydroxycytidine-5'-isopropyl ester; EIDD 2801; MK-4482; Molnupiravir
Appearance:
White to off-white solid.
CAS:
2349386-89-4
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C13H19N3O7/c1-6(2)12(19)22-5-7-9(17)10(18)11(23-7)16-4-3-8(15-21)14-13(16)20/h3-4,6-7,9-11,17-18,21H,5H2,1-2H3,(H,14,15,20)/t7-,9-,10-,11-/m1/s1
InChiKey:
HTNPEHXGEKVIHG-QCNRFFRDSA-N
Long Description:
Chemical. CAS: 2349386-89-4. Formula: C13H19N3O7. MW: 329.3. EIDD-2801 is an orally bioavailable prodrug of the antiviral nucleoside derivative N4-hydroxycytidine (NHC, EIDD-1931).
It is a nucleotide analog inhibitor of RNA-dependent RNA polymerases (RdRps). The compound interferes with the action of viral RNA polymerase. It exerts its antiviral action through introduction of copying errors during viral RNA replication. The active drug incorporates into the genome of RNA viruses, leading to an accumulation of mutations known as viral error catastrophe.
The broad-spectrum antiviral agent EIDD-2801 inhibits viral RNA replication in various unrelated RNA viruses including influenza, Ebola, Venezuelan equine encephalitis virus (VEEV) and coronaviruses, including SARS-CoV, MERS-CoV and SARS-CoV-2.
EIDD-2801 has the potential for COVID-19, seasonal and pandemic influenza treatment.
MDL:
MFCD32663515
Molecular Formula:
C13H19N3O7
Molecular Weight:
329.3
Package Type:
Vial
Product Description:
EIDD-2801 is an orally bioavailable prodrug of the antiviral nucleoside derivative N4-hydroxycytidine (NHC, EIDD-1931). It is a nucleotide analog inhibitor of RNA-dependent RNA polymerases (RdRps). The compound interferes with the action of viral RNA polymerase. It exerts its antiviral action through introduction of copying errors during viral RNA replication. The active drug incorporates into the genome of RNA viruses, leading to an accumulation of mutations known as viral error catastrophe. This mechanism of inhibition of viral replication is also known as lethal mutagenesis. The broad-spectrum antiviral agent EIDD-2801 inhibits viral RNA replication in various unrelated RNA viruses including influenza, Ebola, Venezuelan equine encephalitis virus (VEEV) and coronaviruses, including SARS-CoV, MERS-CoV and SARS-CoV-2. EIDD-2801 has the potential for COVID-19, seasonal and pandemic influenza treatment. Treatment of SARS-CoV-2 infection with EIDD-2801 completely suppressed virus transmission within 24 hours in ferrets.
Purity:
>98% (HPLC)
SMILES:
O=C(N(C=C/1)[C@@H]2O[C@H](COC(C(C)C)=O)[C@@H](O)[C@H]2O)NC1=NO
Solubility Chemicals:
Soluble in DMSO (25mg/ml), DMF (25mg/ml) or water (2mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia: M.Toots, et al.; Sci. Transl. Med. 11, 515 (2019) | New Flu Antiviral Candidate May Thwart Drug Resistance: T. Hampton; JAMA 323, 17 (2020) | Quantitative efficacy paradigms of the influenza clinical drug candidate EIDD-2801 in the ferret model: M. Toots, et al.; Transl. Res. 218, 16 (2020) | An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice: T.P. Sheahan, et al.; Sci. Transl. Med. 12, 541 (2020) | Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model: K. Rosenke, et al.; Nat. Commun. 12, 2295 (2021) | Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets: R.M. Cox, et al.; Nat. Microbiol. 6, 11 (2021) | Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis: F. Kabinger, et al.; Nat. Struct. Mol. Biol. 28, 740 (2021) | Molnupiravir Inhibits Replication of the Emerging SARS-CoV-2 Variants of Concern in a Hamster Infection Model: R. Abdelnabi, et al.; J. Infect. Dis. 224, 749 (2021) | Accelerated first-in-human clinical trial of EIDD-2801/MK-4482 (molnupiravir), a ribonucleoside analog with potent antiviral activity against SARS-CoV-2: W. Holman, et al.; Trials 22, 561 (2021) | Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19: G.R. Painter, et al.; Curr. Opin. Virol. 50, 17 (2021) | Molnupiravir: coding for catastrophe; B. Malone & E.A. Campbell; Nat. Struct. Mol. Biol. 28, 706 (2021)