IAXO-103 (CD14/TLR4 Antagonist) (synthetic)
| Code | Size | Price |
|---|
| IAX-600-003-M001 | 1 mg | £236.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
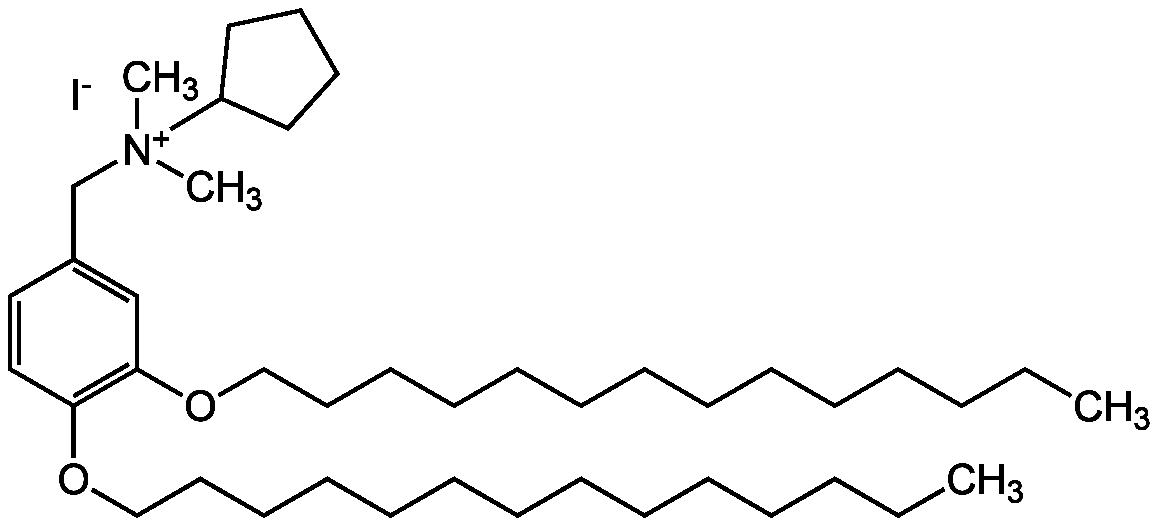
N-(3,4-bis-Tetradecyloxy-benzyl)-N-cyclopentyl-N,N-dimethylammonium iodide
Appearance:
White solid.
Biological Activity:
Described to interfere with human, rat and mouse TLR4/CD14 signaling, other species not tested. Optimal working concentration depends upon the type, purity and concentration and of TLR4 ligand, carrier protein such as LPS-binding protein (LBP), soluble and membrane-bound CD14, the presence of TLR4 co-receptors (e.g. CD36) as well as on type and time of read-out (e.g. cytokine measurement in cell culture supernatant) or the biological outcome of in vivo experiments and therefore needs to be determined for each application. Recommended starting concentration: in vitro: 5µM, in vivo (rodent): 3mg/kg.
CAS:
1202208-36-3
EClass:
32160000
Form (Short):
solid
InChi:
UXOPVNPWTUWUEA-RMKQIWEICG
InChiKey:
InChI=1/C42H78NO2.HI/c1-5-7-9-11-13-15-17-19-21-23-25-29-35-44-41-34-33-39(38-43(3,4)40-31-27-28-32-40)37-42(41)45-36-30-26-24-22-20-18-16-14-12-10-8-6-2;/h33-34,37,40H,5-32,35-36,38H2,1-4H3;1H/q+1;/p-1/fC42H78NO2.I/h;1h/qm;-1
Long Description:
Chemical. CAS: 1202208-36-3. Formula: C42H78INO2. MW: 755.98g/mol (iodide salt). Synthetic. CD14/TLR4 antagonist. Inhibitor of sterile inflammation. Synthetic TLR4/CD14 ligand with TLR4 modulating activities in vitro, and conferring protection against TLR4/CD14-mediated tissue damage and inflammation in vivo. Useful to explore CD14- dependent and TLR4-independent pathways and TLR4 activation by endogenous ligands (e.g. hyaluronic acid oligosaccharides, oxLDL, HMGB1) in sterile inflammation. Shown to inhibit neuropathic pain, secondary necrosis of acute drug-induced liver failure and vascular inflammation, and abdominal aortic aneurysm by blocking non-hematopoietic TLR4 signaling. Useful tool, where inhibition of sterile (auto-) inflammation is desired, without compromising TLR4?s key role in the defense of pathogens.
MDL:
MFCD18382116
Molecular Formula:
C42H78INO2
Molecular Weight:
755.98g/mol (iodide salt)
Other data:
Reconstitution: For a 2mM stock solution, dissolve total vial content in 661µl (1mg size) or 3,306µl (5mg size) DMSO/Ethanol (1:1) (vol:vol).
Package Type:
Plastic Vial
Product Description:
CD14/TLR4 antagonist. Inhibitor of sterile inflammation. Synthetic TLR4/CD14 ligand with TLR4 modulating activities in vitro, and conferring protection against TLR4/CD14-mediated tissue damage and inflammation in vivo. Useful to explore CD14- dependent and TLR4-independent pathways and TLR4 activation by endogenous ligands (e.g. hyaluronic acid oligosaccharides, oxLDL, HMGB1) in sterile inflammation. Shown to inhibit neuropathic pain, secondary necrosis of acute drug-induced liver failure and vascular inflammation, and abdominal aortic aneurysm by blocking non-hematopoietic TLR4 signaling. Useful tool, where inhibition of sterile (auto-) inflammation is desired, without compromising TLR4?s key role in the defense of pathogens.
Purity:
>98% (TLC)
SMILES:
C[N+](C)(C1CCCC1)CC2=CC(OCCCCCCCCCCCCCC)=C(OCCCCCCCCCCCCCC)C=C2.[I-]
Solubility Chemicals:
Soluble in Methanol, DMSO and Ethanol 1:1 (vol:vol): >10mM.
Source / Host:
Synthetic.
Transportation:
Non-Hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C.
References
Inhibition of lipid a stimulated activation of human dendritic cells and macrophages by amino and hydroxylamino monosaccharides: F. Peri, et al.; Angew. Chem.46, 3308 (2007) | TLR4 receptor as new target to treat neuropathic pain: efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice: I. Bettoni, et al.; Glia 56, 1312 (2008) | Glycolipids and benzylammonium lipids as novel antisepsis agents: synthesis and biological characterization: M. Piazza, et al.; J. Med. Chem. 52, 1209 (2009) | Evidence of a specific interaction between new synthetic antisepsis agents and CD14: M. Piazza, et al.; Biochemistry 48, 12337 (2009) | Exploring the LPS/TLR4 signal pathway with small molecules: F. Peri, et al.; Biochem. Soc. Trans. 38, 1390 (2010) (Review) | Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists: F. Peri & M. Piazza; Biotechnol. Adv. 30, 251 (2012) (Review) | Synthetic molecules and functionalized nanoparticles targeting the LPS-TLR4 signaling: A new generation of immunotherapeutics: F. Peri, et al.; Pure Appl. Chem. 84, 97 (2012) (Review) | Toll like receptor 4 antagonist prevents acetaminophen induced acute liver failure in mice: a novel therapeutic strategy: N. Shah, et al.; Gut 61, A28 (2012) | Multivalent glycoconjugates as anti-pathogenic agents. A. Bernardi, et al.; Chem. Soc. Rev. 42, 4709 (2013) (Review) | Toll-like receptor 4 (TLR4) modulation by synthetic and natural compounds: an update: F. Peri & V. Calabrese; Med. Chem. 57, 3612 (2014) (Review) | Human Toll-Like Receptor 4 (hTLR4): Structural and functional dynamics in cancer: V. Mishra & C. Pathak; Int. J. Biol. Macromol. 122, 425 (2019)