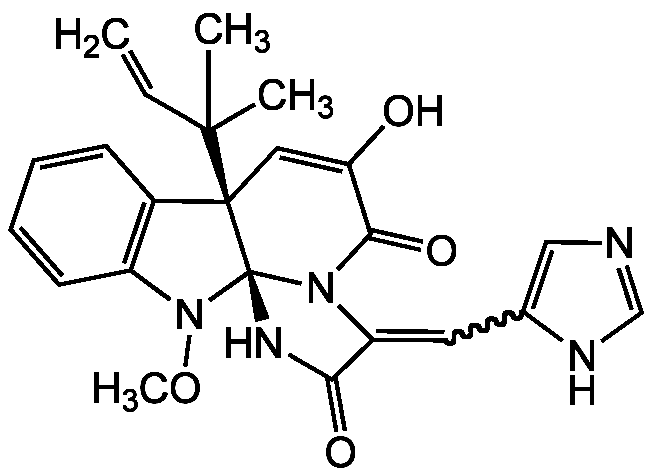
Meleagrin
Product Code: AG-CN2-0451
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0451-M001 | 1 mg | £140.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
6-O-Methyloxaline
Appearance:
Yellow adhered film.
CAS:
71751-77-4
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light.Protect from moisture.
InChi:
InChI=1S/C23H23N5O4/c1-5-21(2,3)22-11-18(29)20(31)27-17(10-14-12-24-13-25-14)19(30)26-23(22,27)28(32-4)16-9-7-6-8-15(16)22/h5-13,29H,1H2,2-4H3,(H,24,25)(H,26,30)/b17-10+/t22-,23-/m0/s1
InChiKey:
JTJJJLSLKZFEPJ-ZAYCRUKZSA-N
Long Description:
Chemical. CAS: 71751-77-4. Formula: C23H23N5O4. MW: 433.5. Isolated from Penicillium sp. Mycotoxin. Alkaloid antibiotic. Bacterial enoyl-acyl carrier protein reductase (FabI) inhibitor. Antineoplastic activity. Moderate cytotoxicity against A-549 and HL-60 cell lines. Induces cell cycle arrest through G2/M phase, presumably by inhibiting tubulin polymerization. Antifouling agent against the barnacle Balanus amphitrite.
MDL:
MFCD08702703
Molecular Formula:
C23H23N5O4
Molecular Weight:
433.5
Package Type:
Vial
Product Description:
Mycotoxin. Alkaloid antibiotic. Bacterial enoyl-acyl carrier protein reductase (FabI) inhibitor. Antineoplastic activity. Moderate cytotoxicity against A-549 and HL-60 cell lines. Induces cell cycle arrest through G2/M phase, presumably by inhibiting tubulin polymerization. Antifouling agent against the barnacle Balanus amphitrite.
Purity:
>90% (HPLC)
SMILES:
CON1C2=C(C=CC=C2)[C@@]2(C=C(O)C(=O)N3C(=CC4=CN=CN4)C(=O)N[C@@]123)C(C)(C)C=C
Solubility Chemicals:
Soluble in DMSO or ethanol.
Source / Host:
Isolated from Penicillium sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Studies on fungal products. VII. The structures of meleagrin and 9-O-p-bromobenzoylmeleagrin: K. Kawai, et al.; Chem. Pharm. Bull. 32, 94 (1984) | Study of the alkaloid composition of the food-infecting penicilles: T.A. Reshetilova, et al.; Food Addit. Contam. 12, 461 (1995) | Production of mycotoxins on artificially and naturally infested building materials: K.F. Nielsen, et al.; Mycopathologia 145, 43 (1999) | Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities: L. Du, et al.; J. Antibiot. 63, 165 (2010) | iTRAQ-based proteomic profiling of the barnacle Balanus amphitrite in response to the antifouling compound meleagrin: Z. Han, et al.; J. Proteome Res. 12, 2090 (2013) | Meleagrin, a new FabI inhibitor from Penicillium chryosogenum with at least one additional mode of action: C.J. Zheng, et al.; PLoS One 8, e78922 (2013)