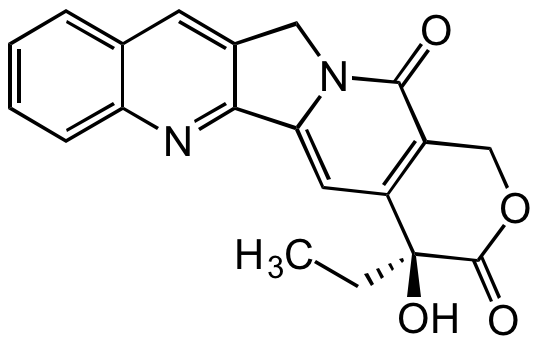
(S)-Camptothecin
Product Code: AG-CN2-0463
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0463-M050 | 50 mg | £35.00 |
Quantity:
| AG-CN2-0463-M250 | 250 mg | £100.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Antibody Isotype: n/a
Antibody Clone: n/a
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
CPT; NSC 94600
Appearance:
White solid.
CAS:
04/03/7689
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Protect from moisture.
Hazards:
H301
InChi:
InChI=1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1
InChiKey:
VSJKWCGYPAHWDS-FQEVSTJZSA-N
Long Description:
Chemical. CAS: 7689-03-4. Formula: C20H16N2O4. MW: 348.4. Isolated from Camptotheca acuminata. Potent anticancer compound. Cell permeable potent DNA topoisomerase I (Topo I) complex inhibitor. Potent apoptosis inducer. Binds reversibly to the DNA topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. The enzyme complex is ubiquinated and destroyed by the 26S proteasome, consequently depleting cellular topoisomerase I. Prevents DNA re-ligation and therefore causes DNA damage which results in apoptosis. Inhibits mitochondrial topoisomerase I (mtTop1). Blocks the cell cycle at low dose and induces apoptosis in a large number of normal and tumor cell lines by cell cycle-dependent and cell cycle-independent processes. Antiprotozoal and antimalarial compound. Inhibitor of HIV replication and of other viruses. Suppresses nitric oxide (NO) biosynthesis. Shown to suppress TNF-alpha-induced expression of the inflammasome and cyclooxygenase 2 (COX-2).
MDL:
MFCD00081076
Molecular Formula:
C20H16N2O4
Molecular Weight:
348.4
Package Type:
Vial
PG:
III
Precautions:
P264, P301, P310, P330
Product Description:
Potent anticancer compound. Cell permeable potent DNA topoisomerase I (Topo I) complex inhibitor. Potent apoptosis inducer. Binds reversibly to the DNA topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. The enzyme complex is ubiquinated and destroyed by the 26S proteasome, consequently depleting cellular topoisomerase I. Prevents DNA re-ligation and therefore causes DNA damage which results in apoptosis. Inhibits mitochondrial topoisomerase I (mtTop1). Blocks the cell cycle at low dose and induces apoptosis in a large number of normal and tumor cell lines by cell cycle-dependent and cell cycle-independent processes. Antiprotozoal and antimalarial compound. Inhibitor of HIV replication and of other viruses. Suppresses nitric oxide (NO) biosynthesis. Shown to suppress TNF-alpha-induced expression of the inflammasome and cyclooxygenase 2 (COX-2).
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
CC[C@@]1(O)C(=O)OCC2=C1C=C1N(CC3=CC4=CC=CC=C4N=C13)C2=O
Solubility Chemicals:
Soluble in DMSO, DMF or MeOH. Insoluble in water
Source / Host:
Isolated from Camptotheca acuminata.
Transportation:
Excepted Quantity
UN Nummer:
UN 2811
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminate: M.E. Wall, et al.; JACS 88, 3888 (1966) | Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I: Y.H. Hsiang, et al.; J. Biol. Chem. 260, 14873 (1985) | On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex: R.P. Hertzberg, et al.; Biochem. 28, 4629 (1989) | Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin: Y.H. Hsiang, et al.; Cancer Res. 49, 5077 (1989) | Irreversible trapping of the DNA-topoisomerase I covalent complex. Affinity labeling of the camptothecin binding site: R.P. Hertzberg, et al.; J. Biol. Chem. 265, 19287 (1990) | Apoptosis induced by Actinomycin D, Camptothecin or Aphidicolin can occur in all phases of the cell cycle: J.M. Glynn, et al.; Biochem. Soc. Trans. 20, 84S (1992) | Camptothecin inhibits Tat-mediated transactivation of type 1 human immunodeficiency virus: C.J. Li, et al.; J. Biol. Chem. 269, 7051 (1994) | Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor, on trypanosomes and Leishmania: A.L. Bodley & T.A. Shapiro; PNAS 92, 3726 (1995) | Human mitochondrial topoisomerase I: H. Zhang, et al.; PNAS 98, 10608 (2001) | Camptothecin suppresses nitric oxide biosynthesis in RAW 264.7 macrophages: W.F. Chiou, et al.; Life Sci. 69, 625 (2001) | Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani: N. Sen, et al.; Cell Death Differ. 11, 924 (2004) | Camptothecin and its analogues: a review on their chemotherapeutic potential: D. Sriram, et al.; Nat. Prod. Res. 19, 393 (2005) (Review) | Review camptothecin: current perspectives: Q.Y. Li, et al.; Curr. Med. Chem. 13, 2021 (2006) (Review) | The effects of camptothecin on RNA polymerase II transcription: roles of DNA topoisomerase I: G. Capranico, et al.; Biochimie 89, 482 (2007) | Camptothecin promotes the production of nitric oxide that triggers subsequent S-nitrosoproteome-mediated signaling cascades in endothelial cells: B. Huang, et al.; Vascul. Pharmacol. 90, 27 (2015) | Freezing Responses in DMSO?Based Cryopreservation of Human iPS Cells: Aggregates vs. Single Cells: R. Li, et al.; Tissue Eng. Part C Methods, 24, 289 (2018)
Related Products
| Product Name | Product Code | Supplier | JC-10 (high purity) | AG-CR1-3600 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|