SUMO2 (human) (rec.) (Rhodamine 110)
| Code | Size | Price |
|---|
| SBB-PS0029-C050 | 50 ug | £415.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Target Species: Human
Shipping:
Dry Ice
Storage:
-80C
Images
Documents
Further Information
Alternate Names/Synonyms:
Small Ubiquitin-related Modifier 2; HSMT3; SMT3 Homolog 2; Sentrin-2; Ubiquitin-like Protein SMT3A
Concentration:
Lot dependent.
EClass:
32160000
Form (Short):
liquid
Formulation:
Liquid. In 50mM HEPES pH 7.5, 100mM sodium chloride.
Handling Advice:
Aliquot to avoid freeze/thaw cycles.Protect from light.
Labels - Conjugates:
Rhodamine
Long Description:
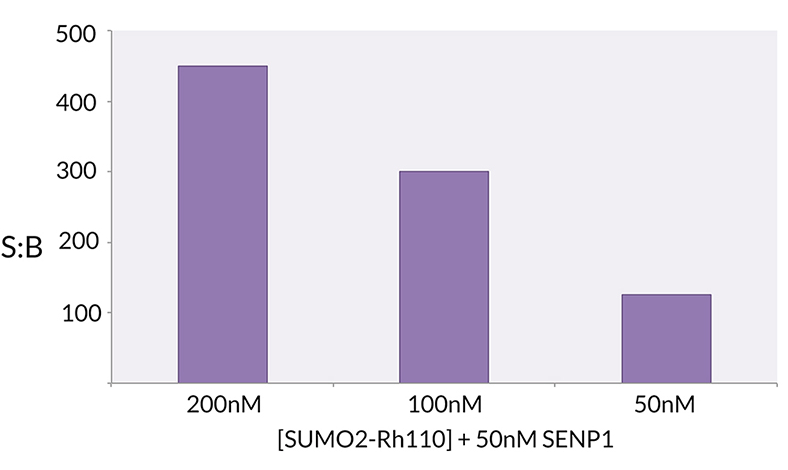
Protein. Human SUMO2 (aa1-93)conjugated at the C-terminus to a quenched Rhodamine 110 dye. Source: E. coli. Formulation: Liquid. In 50mM HEPES pH 7.5, 100mM sodium chloride. Purity: >97% (LCMS). SUMO2 is a Ubiquitin-like protein (UBL) that can be covalently attached to proteins as a monomer or as a lysine-linked polymer. Covalent attachment via an isopeptide bond to its substrates requires prior activation by the E1 complex SAE1-SAE2 and linkage to the E2 enzyme UBE2I, and can be promoted by an E3 ligase such as PIAS1-4, RANBP2, CBX4 or ZNF451. This post-translational modification on lysine residues of proteins plays a crucial role in a number of cellular processes such as nuclear transport, DNA replication and repair, mitosis and signal transduction. This SUMO2 substrate is C-terminally derivatized with a bis-Gly-Rhodamine 110 fluorophore. The bis-Gly-Rh110 is quenched until the amide bond between the C-terminal glycine and the rhodamine compound is hydrolyzed. The efficiency of quenching combined with the powerful signal upon hydrolysis yields an unparalleled signal-to-background. SUMO2-Rh110 can be used to study the deSUMOylating activity of hydrolases SENP1 and SENP2, among other deSUMOylating enzymes. The substrate activity of SUMO2-Rhodamine 110 was determined by measuring the SENP1 catalyzed release of unquenched Gly-Rh-110.
Molecular Weight:
~11kDa
NCBI, Uniprot Number:
P61956
Package Type:
Plastic Vial
Product Description:
SUMO2 is a Ubiquitin-like protein (UBL) that can be covalently attached to proteins as a monomer or as a lysine-linked polymer. Covalent attachment via an isopeptide bond to its substrates requires prior activation by the E1 complex SAE1-SAE2 and linkage to the E2 enzyme UBE2I, and can be promoted by an E3 ligase such as PIAS1-4, RANBP2, CBX4 or ZNF451. This post-translational modification on lysine residues of proteins plays a crucial role in a number of cellular processes such as nuclear transport, DNA replication and repair, mitosis and signal transduction. This SUMO2 substrate is C-terminally derivatized with a bis-Gly-Rhodamine 110 fluorophore. The bis-Gly-Rh110 is quenched until the amide bond between the C-terminal glycine and the rhodamine compound is hydrolyzed. The efficiency of quenching combined with the powerful signal upon hydrolysis yields an unparalleled signal-to-background. SUMO2-Rh110 can be used to study the deSUMOylating activity of hydrolases SENP1 and SENP2, among other deSUMOylating enzymes. The substrate activity of SUMO2-Rhodamine 110 was determined by measuring the SENP1 catalyzed release of unquenched Gly-Rh-110.
Purity:
>97% (LCMS)
Sequence:
Human SUMO2 (aa1-93) (Accession Nr. P61956 http://www.ncbi.nlm.nih.gov/protein/P61956 ) conjugated at the C-terminus to a quenched Rhodamine 110 dye.
Source / Host:
E. coli
Transportation:
Non-hazardous
UNSPSC Category:
Other Proteins
UNSPSC Number:
12352202
Use & Stability:
Stable for at least 1 year after receipt when stored at -80°C.