Anti-SOD1 (Human) mAb
Product Code:
MBL-M062-3
MBL-M062-3
Host Type:
Mouse
Mouse
Antibody Isotype:
IgG1
IgG1
Antibody Clonality:
Monoclonal
Monoclonal
Antibody Clone:
1G2
1G2
Regulatory Status:
RUO
RUO
Target Species:
Human
Human
Application:
Western Blot (WB)
Western Blot (WB)
Shipping:
4°C
4°C
Storage:
4°C
4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| MBL-M062-3 | 100 ug | £323.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: United States.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Applications:
WB - 1 ug/mL (chemiluminescence detection system)
Background:
Cu/Zn-superoxide dismutases (Cu/Zn-SOD, SOD1) are metalloenzymes involved in the mechanisms of cellular defense against oxidative damage. They have been found in the cytoplasm of all the eukaryotic cells and in the periplasm of several bacterial species. Human SOD1 (approx. MW=19 kDa) is homodimers that contain one atom of zinc and one atom of copper per subunit and catalyze the dismutation of the superoxide anion (O2 -
?) quite efficiently at a rate close to the diffusion limit, over the entire pH range from 5 to 10.: 2O2 -?+2H+?O2+H2O2 (SOD1) SOD1 possess a very compact and stable structure that is highly resistant to urea, SDS and proteolytic enzymes. SOD1 homodimer consist of two pieces of eight-stranded β-barrel structure monomer close packaged on each hydrophobic interface stabilized by intrasubunit disulfide bond. SOD1 catalyzes the disproportion of superoxide anions via the alternate reduction and reoxidation of the copper ion at the active site of the enzyme. Recent studies in yeast have identified a protein termed the copper chaperone for superoxide dismutase (CCS) as the factor responsible for copper incorporation into SOD1 through direct protein-protein interaction. SOD1's endogenous role remains poorly understood. Mice lacking this enzyme were apparently healthy and displayed no increased sensitivity to hyperoxia. However, they exhibited a pronounced susceptibility to paraquat which induce acute oxidative stress in mice. As a relation to disease, elevated level of SOD1 is reported in Down syndrome patients as a result of increase of genedosage (trisomy). Moreover, linkage studies have revealed that mutations in SOD1 are responsible for 10-15% of case of the fetal motor neuron disease familial amyotrophic lateral sclerosis (FALS). Evidence from transgenic mice expressing FALS associated SOD1 mutations, as well as mice homozygous for a deletion of the SOD1 gene, indicates that this neuronal degeneration arises from a gain-of-function associated with the SOD1 mutations rather than loss-of-function. In addition to the legitimate dismutation reaction, SOD1 is able to catalyze surrogate reactions such as the production of ?OH using anionic scavengers and H2O2 and nitration of the tyrosine residues of proteins by peroxynitrate. These reactions might be the causes of cytotoxicity.
Concentration:
1 mg/mL
Formulation:
This antibody is lyophilized form. Prepare a stock solution by dissolving the lyophilized antibody with 100 mL of distilled water. After reconstitution, IgG concentration should be 1 mg/mL in PBS (pH 7.2)/1% Sucrose. No preservative is contained.
Gene IDs:
Human: 6647 Mouse: 20655
Immunogen Translated:
Full-length recombinant human SOD1
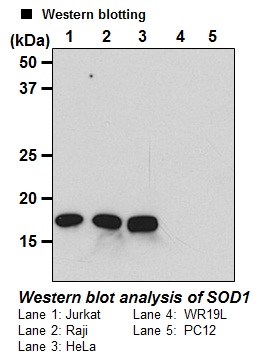
Reactivity:
This antibody detects ~20 kDa of human
SOD1 on Western blotting with total cell lysate from
human cell lines.
Shelf Life:
1 year
Source:
This antibody was purified from mouse ascites
fluid using protein A agarose. This hybridoma was
established by fusion of mouse myeloma cell Sp2/0 with
Balb/c mouse splenocyte immunized with the recombinant
full-length human SOD1.
Target:
SOD1
Documents
References
1) Schonhoff, C. M., et al., PNAS 103, 2404-2409 (2006)
2) Kanekura, K., et al., J. Biol. Chem. 279, 19247-19256 (2004)
3) Hashimoto, Y., et al., J. Biol. Chem. 279, 16767-16777 (2004)
4) Hashimoto, Y., et al., PNAS 98, 6336-6341 (2001)
5) Casareno, R.L.B., et al., J. Biol. Chem. 273, 23625-28 (1998)
6) Przedborski, S., et al., Nat. Genet. 18, 99-100 (1998)
7) Fridovich, I., et al., J. Biol. Chem. 272, 18515-17 (1997)
8) Kondo, T., et al., J. Neurosci. 17, 4180-89 (1997)
9) Yim, M. B., et al., PNAS 93, 5709-14 (1996)
10) Reaume, A. G., et al., Nat. Genet. 13, 43-47 (1996)
11) Brown, R. H., Jr., et al., Cell 80, 687-692 (1995)
12) Fridovich, L., et al., Annu. Rev. Biochem. 64, 97-112 (1995)
13) Rosen, D. R., et al., Nature 362, 59-62 (1993)
14) Deng, H. X., et al., Science 261, 1047-1051 (1993)
15) Badway, J. A., et al.,Annu. Rev. Biochem. 49, 695-726 (1980)