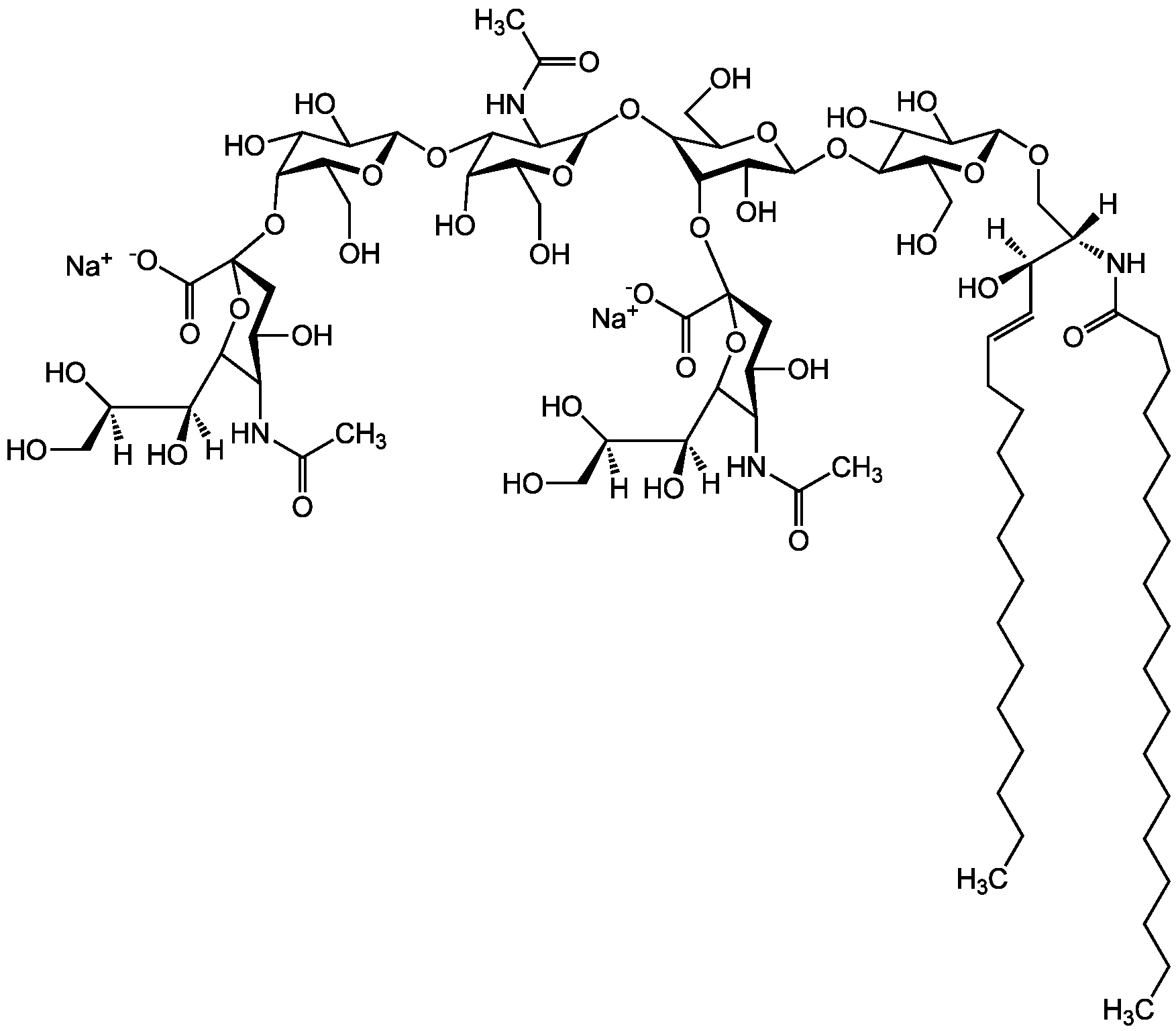
Ganglioside GD1a . disodium salt (bovine brain)
Product Code:
AG-CN2-9003
AG-CN2-9003
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CN2-9003-M001 | 1 mg | £95.00 |
Quantity:
| AG-CN2-9003-M005 | 5 mg | £290.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
GD1a . 2Na; Disialoganglioside GD1a . 2Na
CAS:
12707-58-3
EClass:
32160000
Endotoxin:
Not detectable.
Form (Short):
liquid
Formulation:
Lyophilized.
Handling Advice:
Hygroscopic.Protect from moisture.
InChi:
InChI=1S/C84H148N4O39.2Na/c1-6-8-10-12-14-16-18-20-21-23-25-27-29-31-33-35-58(103)88-48(49(98)34-32-30-28-26-24-22-19-17-15-13-11-9-7-2)44-116-78-67(109)65(107)70(55(41-92)118-78)121-80-69(111)76(127-84(82(114)115)37-51(100)60(86-46(4)96)75(126-84)63(105)53(102)39-90)72(57(43-94)120-80)122-77-61(87-47(5)97)73(64(106)54(40-91)117-77)123-79-68(110)66(108)71(56(42-93)119-79)124-83(81(112)113)36-50(99)59(85-45(3)95)74(125-83)62(104)52(101)38-89;;/h32,34,48-57,59-80,89-94,98-102,104-111H,6-31,33,35-44H2,1-5H3,(H,85,95)(H,86,96)(H,87,97)(H,88,103)(H,112,113)(H,114,115);;/q;2*+1/p-2/b34-32+;;/t48-,49+,50?,51?,52+,53+,54?,55?,56?,57?,59+,60+,61?,62-,63-,64-,65?,66?,67?,68?,69?,70+,71-,72-,73?,74?,75?,76?,77+,78+,79-,80-,83+,84-;;/m0./s1
InChiKey:
RLDNBFLGJKPAGV-GLORMQLTSA-L
Long Description:
Chemical. CAS: 12707-58-3. Formula: C84H146N4O39 . 2Na. MW: 1836.1 . 46.0 (calculated on sphingosine C18:1 and stearic acid). Isolated from bovine brain. Gangliosides are acidic glycosphingolipids that form lipid rafts in the outer leaflet of the cell plasma membrane, especially in neuronal cells in the central nervous system. They participate in cellular proliferation, differentiation, adhesion, signal transduction, cell-to-cell interactions, tumorigenesis and metastasis. The accumulation of gangliosides has been linked to several diseases. Ganglioside GD1a is the major ganglioside of the nervous system. It is converted to GM1 by bacterial, viral and mammalian sialidases. It is a differentiation marker for cell growth.
MDL:
MFCD00130960
Molecular Formula:
C84H146N4O39 . 2Na
Molecular Weight:
1836.1 . 46.0 (calculated on sphingosine C18:1 and stearic acid)
Package Type:
Glass Vial
Product Description:
Gangliosides are acidic glycosphingolipids that form lipid rafts in the outer leaflet of the cell plasma membrane, especially in neuronal cells in the central nervous system. They participate in cellular proliferation, differentiation, adhesion, signal transduction, cell-to-cell interactions, tumorigenesis and metastasis. The accumulation of gangliosides has been linked to several diseases. Ganglioside GD1a is the major ganglioside of the nervous system. It is converted to GM1 by bacterial, viral and mammalian sialidases. It is a differentiation marker for cell growth.
Purity:
>98% (TLC)
Sequence:
Structure: IV3Neu5AcII3NeuAcGgOse4Cer; alpha-Neu5Ac-(2-3)-beta-Gal-(1-3)-beta-GalNAc-(1-4)-[alpha-Neu5Ac-(2-3)-]beta-Gal-(1-4)-beta-Glc-(1-1)-Cer; Cer: Sphingosine C18:1-C20:1, ~1:1 to 1:3 by vol.; stearic acid over 90%
SMILES:
[Na+].[Na+].[H][C@@](O)(CO)[C@]([H])(O)C1O[C@@](CC(O)[C@H]1NC(C)=O)(O[C@@H]1C(O)C(O)[C@H](OC2[C@@H](O)[C@H](CO)O[C@H](O[C@H]3C(CO)O[C@@H](O[C@H]4C(O)C(O)[C@H](OC[C@]([H])(NC(=O)CCCCCCCCCCCCCCCCC)[C@]([H])(O)C=CCCCCCCCCCCCCC)O[C@H]4CO)C(O)[C@H]3O[C@@]3(CC(O)[C@@H](NC(C)=O)C(O3)[C@@]([H])(O)[C@]([H])(O)CO)C([O-])=O)C2NC(C)=O)O[C@H]1CO)C([O-])=O
Solubility Chemicals:
Soluble in water (micellar aggregates) or chloroform:methanol (2:1).
Source / Host:
Isolated from bovine brain.
Transportation:
Non-hazardous
UNSPSC Category:
Glycolipids/Phospholipids/Sphingolipids
UNSPSC Number:
12352211
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Role of membrane gangliosides in the binding and action of bacterial toxins: P.H. Fishman; J. Membr. Biol. 69, 85 (1982) | Dynamic and structural properties of sphingolipids as driving force to the formation of membrane domains: S. Sonnino, et al.; Chem. Rev. 106, 2111 (2006) | The conserved arginine residue in all siglecs is essential for Siglec-7 binding to sialic acid: A. Yoshimura, et al.; BBRC in press, (2020)
Related Products
| Product Name | Product Code | Supplier | Ganglioside GM1 . sodium salt (bovine brain) | AG-CN2-9000 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ganglioside GQ1b . tetrasodium salt (bovine brain) | AG-CN2-9007 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||