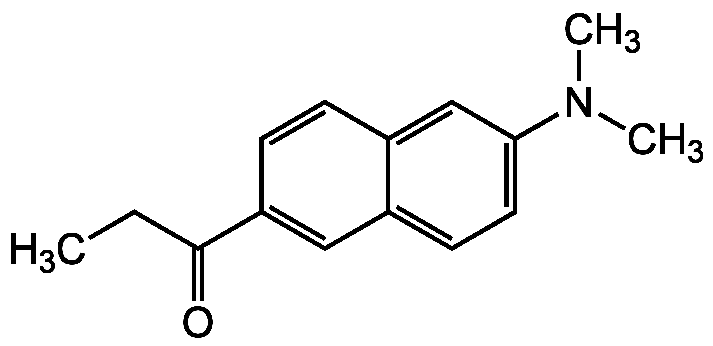
N,N-Dimethyl-6-propionyl-2-naphthylamine
Product Code:
CDX-D0077
CDX-D0077
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
-20 °C
-20 °C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-D0077-M025 | 25 mg | £117.00 |
Quantity:
| CDX-D0077-M050 | 50 mg | £175.00 |
Quantity:
| CDX-D0077-M500 | 500 mg | £1,208.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Prodan
Appearance:
Light yellow powder.
CAS:
70504-01-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C15H17NO/c1-4-15(17)13-6-5-12-10-14(16(2)3)8-7-11(12)9-13/h5-10H,4H2,1-3H3
InChiKey:
MPPQGYCZBNURDG-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 70504-01-7. Formula: C15H17NO. MW: 227.3. Synthetic. N,N-dimethyl-6-propionyl-2-naphthylamine (prodan) has both an electron-donor and an electron-acceptor substituent, resulting in a large excited-state dipole moment and extensive solvent polarity-dependent fluorescence shifts. When prodan is incorporated into membranes, their fluorescence spectra are sensitive to the physical state of the surrounding phospholipids. In membranes, prodan appears to localize at the surface, although Fourier transform infrared measurements indicate some degree of penetration into the lipid interior. Excited-state relaxation of prodan is sensitive to the nature of the linkage between phospholipid hydrocarbon tails and the glycerol backbone. Tubulin and its hydrophobic surfaces have been probed with the enviroment-sensitive probes prodan. Prodan is also used for the generation of peroxy-oxalate chemiluminescence with H2O2.
MDL:
MFCD00056615
Molecular Formula:
C15H17NO
Molecular Weight:
227.3
Package Type:
Vial
Product Description:
N,N-dimethyl-6-propionyl-2-naphthylamine (prodan) has both an electron-donor and an electron-acceptor substituent, resulting in a large excited-state dipole moment and extensive solvent polarity-dependent fluorescence shifts. When prodan is incorporated into membranes, their fluorescence spectra are sensitive to the physical state of the surrounding phospholipids. In membranes, prodan appears to localize at the surface, although Fourier transform infrared measurements indicate some degree of penetration into the lipid interior. Excited-state relaxation of prodan is sensitive to the nature of the linkage between phospholipid hydrocarbon tails and the glycerol backbone. Tubulin and its hydrophobic surfaces have been probed with the enviroment-sensitive probes prodan. Prodan is also used for the generation of peroxy-oxalate chemiluminescence with H2O2.
Purity:
>98% (HPLC)
SMILES:
CCC(=O)C1=CC2=CC=C(C=C2C=C1)N(C)C
Solubility Chemicals:
Soluble in methanol, DMF, acetonitrile or acetone.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) B.A. Rowe et al.; J. Phys. Chem. B 110(30), 15021 (2006) | (2) E. Omanovic et al.; Int. J. Environm. Anal. Chem. 85(12-13), 853 (2005) | (3) M. Lin et al.; C. Huie; Anal. Chim. Acta 339(1-2), 131 (1997) | (4) A. Chakrabarti et al.; Biochem. Biophys. Res. Commun. 226, 495 (1996) | (5) P.L.G. Chong et al.; Biochemistry 28, 8358 (1989) | (6) P.L.G. Chong et al.; Biochemistry 27, 399 (1988)
Related Products
| Product Name | Product Code | Supplier | 2',7'-Dichlorofluorescein | CDX-D0004 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-[4'-(2'-Methyl)thiazolyl]phenol | CDX-M0006 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||