SB415286
Product Code:
AG-CR1-3658
AG-CR1-3658
Regulatory Status:
RUO
RUO
Shipping:
-20°C
-20°C
Storage:
Short Term: +4°C. Long Term: -20°C
Short Term: +4°C. Long Term: -20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CR1-3658-M001 | 1 mg | £45.00 |
Quantity:
| AG-CR1-3658-M005 | 5 mg | £105.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
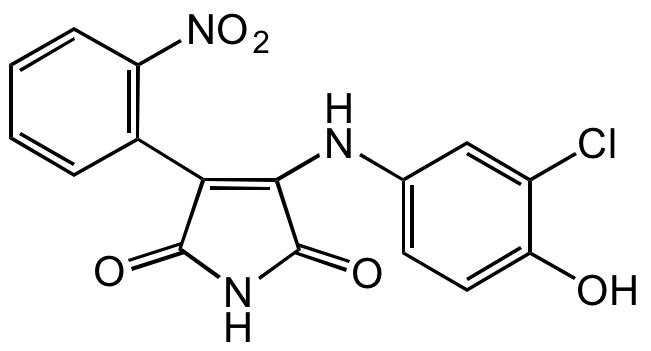
3-[(3-Chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione
Appearance:
Yellow solid.
CAS:
264218-23-7
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C16H10ClN3O5/c17-10-7-8(5-6-12(10)21)18-14-13(15(22)19-16(14)23)9-3-1-2-4-11(9)20(24)25/h1-7,21H,(H2,18,19,22,23)
InChiKey:
PQCXVIPXISBFPN-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 264218-23-7. Formula: C16H10ClN3O5. MW: 359.7. Synthetic. Potent and selective cell permeable, ATP-competitive inhibitor of GSK3alpha with an IC50 value of 78nM (and similar potency for GSK3beta). DYRK1A inhibitor (IC50=0.9µM). Stimulates glycogen synthesis in liver cells and induces beta-catenin-dependent gene transcription. Acts as neuroprotectant and prevents neuronal cell death induced by PI3-kinase pathway. Anticancer agent with antiproliferative activity. Glycogen synthase kinase 3 (GSK3) is a serine/threonine protein kinase that is inhibited by an assortment of extracellular stimuli such as insulin, growth factors, cell specification factors and cell adhesion. Its activity regulates many cell functions including the control of cell division, apoptosis and inflammation.
MDL:
MFCD04039789
Molecular Formula:
C16H10ClN3O5
Molecular Weight:
359.7
Package Type:
Plastic Vial
Precautions:
P302, P352, P304, P340, P305, P351, P338
Product Description:
Potent and selective cell permeable, ATP-competitive inhibitor of GSK3alpha with an IC50 value of 78nM (and similar potency for GSK3beta). DYRK1A inhibitor (IC50=0.9µM). Stimulates glycogen synthesis in liver cells and induces beta-catenin-dependent gene transcription. Acts as neuroprotectant and prevents neuronal cell death induced by PI3-kinase pathway. Anticancer agent with antiproliferative activity. Glycogen synthase kinase 3 (GSK3) is a serine/threonine protein kinase that is inhibited by an assortment of extracellular stimuli such as insulin, growth factors, cell specification factors and cell adhesion. Its activity regulates many cell functions including the control of cell division, apoptosis and inflammation.
Purity:
>97%
Signal word:
Warning
SMILES:
ClC1=CC(NC2=C(C3=C([N+]([O-])=O)C=CC=C3)C(NC2=O)=O)=CC=C1O
Solubility Chemicals:
Soluble in DMSO (10mg/ml), ethanol (5mg/ml) or methanol.
Transportation:
Non-hazardous
UNSPSC Category:
Protein Kinase Modulators
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Selective small molecule inhibitors or glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription: M.P. Coghlan, et al.; Chem. & Biol. 7, 793 (2000) | Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurons from death: D.A.E. Cross, et al.; J. Neurochem. 77, 94 (2001) | Use of lithium and SB-415286 to explore the role of glycogen synthase kinase-3 in the regulation of glucose transport and glycogen synthase: K. MacAulay, et al.; Eur. J. Biochem. 270, 3829 (2003) | GSK3 inhibitors: development and therapeutic potential: P. Cohen & M. Goedert; Nat. Rev. Drug Discov. 3, 479 (2004) | Reduction of experimental colitis in the rat by inhibitors of glycogen synthase kinase-3beta: B.J.R. Whittle, et al.; Br. J. Pharmacol. 147, 575 (2006) | The selectivity of protein kinase inhibitors: a further update: J. Bain, et al.; Biochem. J. 408, 297 (2007) | Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS: J. Dill, et al.; J. Neurosci. 28, 8914 (2008) | A molecular study of pathways involved in the inhibition of cell proliferation in neuroblastoma B65 cells by the GSK-3 inhibitors lithium and SB-415286: J.G. Pizarro, et al.; J. Cell. Mol. Med. 13, 3906 (2009)