Showing 25 of 751 items meeting your criteria.
| | Type | Category | Supplier | |
|---|
| |
 | Highlights and best sellers of Elabscience metabolism assay kits |
Documents | Metabolism | Elabscience | |
 | Apoptosis Assay, Cell Proliferation and Toxicity Assay, Cell Cycle Assay. Meet your various experimental requirements. |
Documents | Cell Based Assays | Elabscience | |
 | CCK-8 is a kit for detecting cell viability based on WST-8. The detection principle is that WST-8 can be reduced to water-soluble methoxly product under the action of electron carrier 1-methoxy (PMS), and the amount of methylate is directly proportional to the number of living cells. CCK-8 solution can be directly added to the cells, and the orange methyl dye generated can be dissolved in the tissue culture medium. The number of cells in the culture medium can be calculated according to the standard curve by detecting the light absorption near 450 nm. |
Documents | Cell Based Assays | Elabscience | |
 | Contents: 1. Metabolism Assay Kits 2. Product advantage 3. Oxidative stress 3.1. oxidative stress assay 3.2.1. reactive oxygen species 3.2.2. antioxidant capacity 3.2.3. oxidation products 4. Publications 5. References 6. Products explanation |
Documents | Metabolism | Elabscience | |
 | Accelerate Your Biochemical Experiments with Our Superior Biochemical Kits. Contents: Highlights of Elabscience Biochemical Kits; Elabscience Extensive Biochemical Kits; Best Sellers of Biochemical Kits; Publications Citing Use of Elabscience Biochemical Kits |
Documents | Metabolism | Elabscience | |
 | Early in apoptosis, before the loss of membrane integrity, PS translocates from the inner to the outer leaflet of the plasma membrane, exposing it to the external cellular environment at the surface of the plasma membrane. Annexin V is a 35-36 kDa, calcium-dependent, phospholipid-binding protein with high affinity for PS and is used to indirectly monitor PS translocation. Elabscience Annexin V Apoptosis Detection Kits have 15 fluorochromes and 3 nucleic acid dyes for multiple research requirements. |
Documents | Cell Based Assays | Elabscience | |
 | Contents: ELISA Kits Characteristic Advantages, Why Choose Elabscience® ELISA Kits, MS ELISA Kit Product Features, QuicKey Pro™ ELISA Kit Product Features, Top-30 Selling ELISA Kits, and High IF Literature. |
Documents | ELISA | Elabscience | |
 | YAP1 encodes the human ortholog of chicken YAP protein which binds to the SH3 domain of the Yes proto-oncogene product. This protein contains a WW domain that is found in various structural, regulatory and signaling molecules in yeast, nematode, and mammals, and may be involved in protein-protein interaction. |
Documents | Antibodies | Abnova Corporation | |
 | SQSTM1 encodes a multifunctional protein that binds ubiquitin and regulates activation of the nuclear factor kappa-B (NF-kB) signaling pathway. The protein functions as a scaffolding/adaptor protein in concert with TNF receptor-associated factor 6 to mediate activation of NF-kB in response to upstream signals. Alternatively spliced transcript variants encoding either the same or different isoforms have been identified for this gene. Mutations in this gene result in sporadic and familial Paget disease of bone. |
Documents | Antibodies | Abnova Corporation | |
 | High purity, Animal free, Quality assured, Cost-effective, Save more with bulk order. Abnova, March 2023 |
Documents | Proteins | Abnova Corporation | |
 | High purity, Animal free, Quality assured, Cost-effective, Save more with bulk order. Popular Recombinant & Native Proteins from Abnova, April 2023 |
Documents | Proteins | Abnova Corporation | |
 | MIF encodes a lymphokine involved in cell-mediated immunity, immunoregulation, and inflammation. It plays a role in the regulation of macrophage function in host defense through the suppression of anti-inflammatory effects of glucocorticoids. This lymphokine and the JAB1 protein form a complex in the cytosol near the peripheral plasma membrane, which may indicate an additional role in integrin signaling pathways. |
Documents | Antibodies | Abnova Corporation | |
 | Datasheet for product P0001015 |
Documents | Datasheets | Addex Bio | |
 | Cloud-Clone Active proteins based on GMP level development, can provide a variety of specifications of protein, to meet the needs of basic science experiments, drug screening, animal drug delivery and other experiments. High Purity, Multi-species, Multi-specifications, Multi-detections |
Documents | Proteins | Cloud-Clone Corp. | |
 | After 14 years of development and technology accumulation, Cloud-Clone has developed ELISA kit and CLIA kit based on enzyme linked immunoassay technology. 8,000+ kinds of high quality assay kits covering 2,000+ indicators and 10+ species are designed to assist your experimental research. Includes Sandwich ELISA Kit, Competition ELISA Kit, CLIA Kit, Antibody ELISA, Mini ELISA, Instant ELISA, High-sensitive ELISA, Wide-range ELISA. |
Documents | ELISA | Cloud-Clone Corp. | |
| How do you operate ELISA kits and get a satisfactory test result? What precautions should be paid attention to?
Check this video recorded in ELK Biotechnologys lab! |
Videos | ELISA | ELK Biotechnology | |
| ELISA experiment operation video |
Videos | ELISA | ELK Biotechnology | |
| ELK Biotechnology CO., LTD is manufacturer of ELISA kits, Molecular biology products and Antibodies. |
Videos | | ELK Biotechnology | |
 | The CCK-8 kit has been cited in research paper published in journals with high impact factor. Excellent choice for cell activity and cytotoxicity detection. 41.444 highest impact factor! |
Documents | Cell Based Assays | Elabscience | |
 | 1. ELISA and Metabolism Assay Kit: IF=66.85. 2. Flow Cytometry Antibody IF=27.521. 3. Apoptosis Kit: IF=11.092. 4. Antibody: IF10.75 |
Documents | | Elabscience | |
 | ELISA Kits, Antibodies, Molecular biology: Full range of products, fast delivery, strict quality control, high stability and specificity, reliable technical support and better after-sales service, cost effective. |
Documents | ELISA | ELK Biotechnology | |
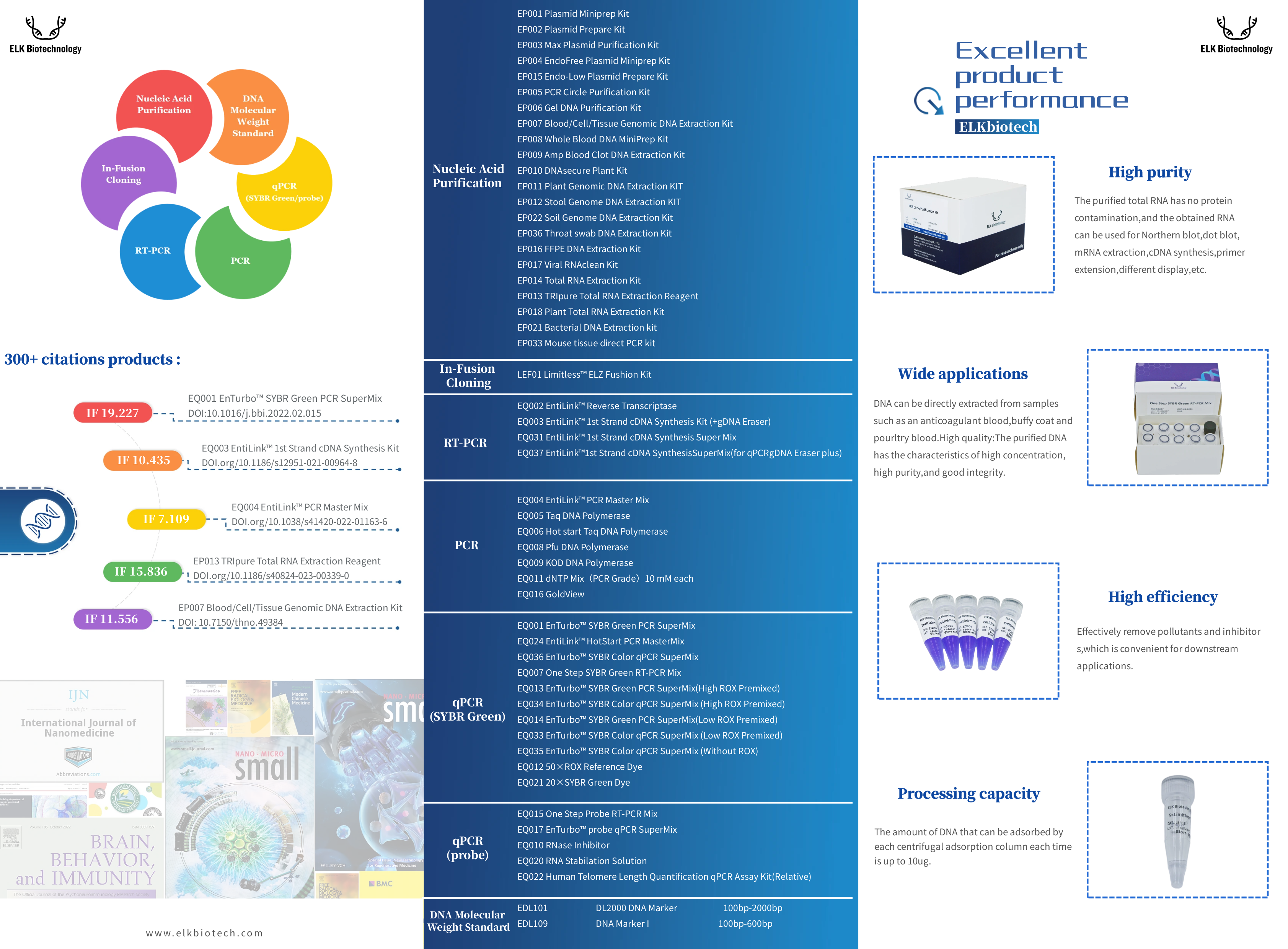 | Molecular Products 300+ Citation from ELK Biotechnology: nucleic acid purification, in-fusion cloning, RT-PCR, PCR, qPCR (SYBR Green), qPCR probe |
Documents | Molecular Biology | ELK Biotechnology | |
 | 8500+ ELISA Kits from ELK Biotechnology: high sensitivity, high specificity, excellent recovery rate. |
Documents | ELISA | ELK Biotechnology | |
 | MBL offers ELISA kits for the detection and monitoring of ABDs. |
Documents | Autoimmune Diseases | MBL | |
 | Through structural analysis and screening of 2342 marketed drugs and 1487 clinical phase drugs, TargetMol has constructed a Drug-Fragment Library consisting of 1159 fragments. |
Documents | Small Molecules | TargetMol | |