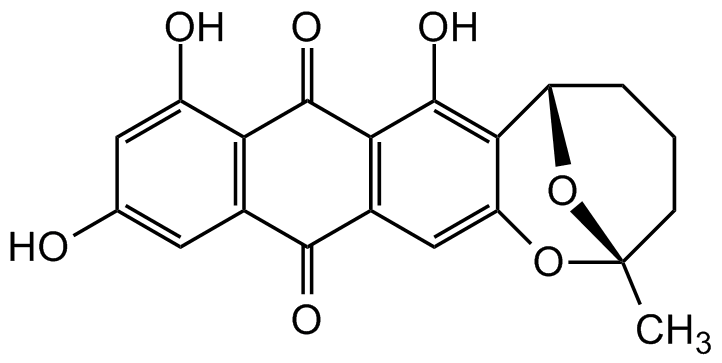
Averufin
Product Code: AG-CN2-0527
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0527-M001 | 1 mg | £105.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(2S,6S)-7,9,11-Trihydroxy-2-methyl-3,4,5,6-tetrahydro-2H-2,6-epoxyanthra[2,3-b]oxocine-8,13-dione; BRN1299991
Appearance:
Yellow to orange solid.
CAS:
14016-29-6
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302, H312, H319
InChi:
InChI=1S/C20H16O7/c1-20-4-2-3-12(26-20)16-13(27-20)7-10-15(19(16)25)18(24)14-9(17(10)23)5-8(21)6-11(14)22/h5-7,12,21-22,25H,2-4H2,1H3/t12-,20-/m0/s1
InChiKey:
RYFFZJHGQCKWMV-YUNKPMOVSA-N
Long Description:
Chemical. CAS: 14016-29-6. Formula: C20H16O7. MW: 368.3. Isolated from fungus Aspergillus versicolor. Mycotoxin. Biosynthetic precursor of aflatoxins. Antibiotic. Shows moderate antibacterial activity against Gram-positive bacteria. Oxidative phosphorylation (OXPHOS) inhibitor. Inhibits succinate cytochrome c reductase complex (III) of the respiratory chain in mitochondria. Useful tool for immunometabolism research.
MDL:
MFCD01729505
Molecular Formula:
C20H16O7
Molecular Weight:
368.3
Package Type:
Vial
Precautions:
P270, P280, P301+P312, P302+P352, P312
Product Description:
Mycotoxin. Biosynthetic precursor of aflatoxins. Antibiotic. Shows moderate antibacterial activity against Gram-positive bacteria. Oxidative phosphorylation (OXPHOS) inhibitor. Inhibits succinate cytochrome c reductase complex (III) of the respiratory chain in mitochondria. Useful tool for immunometabolism research.
Purity:
>95%
Signal Word:
Warning
SMILES:
OC1=CC(O)=CC(C(C2=C3C(O)=C4C(O[C@@]5(C)CCC[C@@H]4O5)=C2)=O)=C1C3=O
Solubility Chemicals:
Soluble in DMSO (5mg/ml) or methanol (1mg/ml).
Source / Host:
Isolated from fungus Aspergillus versicolor.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
The Inhibition of Mitochondrial Respiration by Anthraquinone Mycotoxiins, Averufin and Versicolorins A, B: K. Kawai, et al.; Proc. Jpn. Assoc. Mycotoxicol. 1983, 35 (1983) | Averufin, an anthraquinone mycotoxin possessing a potent uncoupling effect on mitochondrial respiration: K. Kawai, et al.; Appl. Environ. Microbiol. 47, 481 (1984) | Genotoxicity of a variety of mycotoxins in the hepatocyte primary culture/DNA repair test using rat and mouse hepatocytes: H. Mori, et al.; Cancer Res. 44, 2918 (1984) | Averufanin is an aflatoxin B1 precursor between averantin and averufin in the biosynthetic pathway: S.P. McCormick, et al.; Appl. Environ. Microbiol. 53, 14 (1987) | Monoamine oxidase inhibitors from a fungus: Emericella navahoensis: M. Yamazaki, et al.; Chem. Pharm. Bull. 36, 670 (1988) | Isolation, structure elucidation and biological activity of 8-O-methylaverufin and 1,8-O-dimethylaverantin as new antifungal agents from Penicillium chrysogenum: R.P. Maskey, et al.; J. Antibiot. 56, 459 (2003) | The antifungal metabolites obtained from the rhizospheric Aspergillus sp. YIM PH30001 against pathogenic fungi of Panax notoginseng: K. Liu, et al.; Nat. Prod. Res. 28, 2334 (2014) | Induction of Secondary Metabolites from the Marine-Derived Fungus Aspergillus versicolor through Co-cultivation with Bacillus subtilis: N.M. Abdel-Wahab, et al.; Planta Med. 85, 503 (2019)