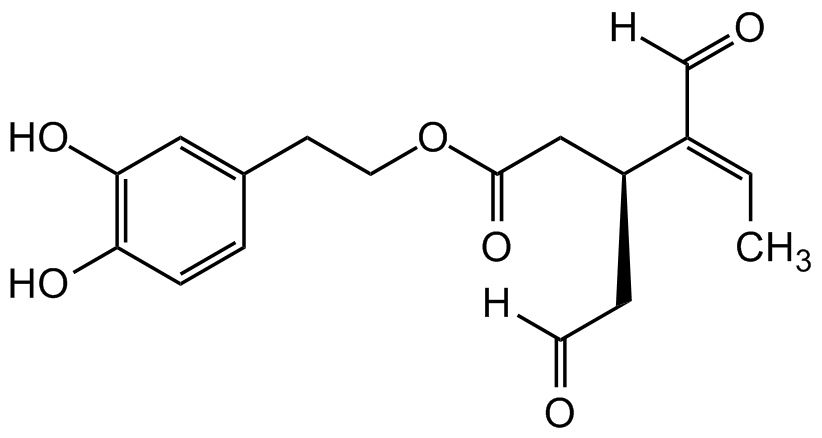
Oleacein
Product Code: AG-CN2-0535
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0535-M005 | 5 mg | £220.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +4°C Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
3,4-DHPEA-EDA; 3,4-Dihydroxyphenylethanol-elenolic acid dialdehyde; Oleacin
Appearance:
Yellow to amber oil.
CAS:
149183-75-5
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C17H20O6/c1-2-13(11-19)14(5-7-18)10-17(22)23-8-6-12-3-4-15(20)16(21)9-12/h2-4,7,9,11,14,20-21H,5-6,8,10H2,1H3/b13- 2-/t14-/m0/s1
InChiKey:
XLPXUPOZUYGVPD-VMPILDALSA-N
Long Description:
Chemical. CAS: 149183-75-5. Formula: C17H20O6. MW: 320.3. Oleacin is a phenolic compound of extra virgin olive oil and has antioxidant, anti-inflammatory, antitumor, antiproliferative, antimicrobial, cardioprotective, antiobesity and antiatherosclerotic properties. In vitro studies showed that oleacein reduces ROS production by f-MLP and PMA-stimulated neutrophils (inhibiting the release of myeloperoxidase). 5-Lipoxygenase (5-LOX) and NF-kappaB signaling inhibitor. Improves insulin sensitivity in obese mice. Dual substrate-inhibitor of catechol-O-methyltransferase. Inhibits the catalytic activities of nicotinamide N-methyltransferase, ATP-citrate lyase, lysine-specific demethylase 6A and N-methyltransferase 4.
MDL:
MFCD09837704
Molecular Formula:
C17H20O6
Molecular Weight:
320.3
Other data:
Note: Shown to react spontaneously with water or methanol to give mixtures of hemiacetals or acetals (see Karkoula et al 2012).
Package Type:
Vial
Product Description:
Oleacin is a phenolic compound of extra virgin olive oil and has antioxidant, anti-inflammatory, antitumor, antiproliferative, antimicrobial, cardioprotective, antiobesity and antiatherosclerotic properties. In vitro studies showed that oleacein reduces ROS production by f-MLP and PMA-stimulated neutrophils (inhibiting the release of myeloperoxidase). 5-Lipoxygenase (5-LOX) and NF-kappaB signaling inhibitor. Improves insulin sensitivity in obese mice. Dual substrate-inhibitor of catechol-O-methyltransferase. Inhibits the catalytic activities of nicotinamide N-methyltransferase, ATP-citrate lyase, lysine-specific demethylase 6A and N-methyltransferase 4.
Purity:
>97% (HPLC)
SMILES:
OC1=C(O)C=CC(CCOC(C[C@H](CC([H])=O)/C(C([H])=O)=CC)=O)=C1
Solubility Chemicals:
Soluble in DMSO, dichloromethane or acetonitrile. Slightly soluble in methanol, ethanol or water (0.1mg/ml).
Source / Host:
Isolated from Olea europaea.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Simple and hydrolyzable compounds in virgin olive oil. 3. Spectroscopic characterizations of the secoiridoid derivatives: G. Montedoro, et al.; J. Agric. Food Chem. 41, 2228 (1993) | Isolation of an angiotensin converting enzyme (ACE) inhibitor from Olea europaea and Olea lancea: K. Hansen, et al.; Phytomedicine 2, 319 (1996) | Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity: E. Medina, et al.; J. Agr. Food Chem. 54, 4954 (2006) | Direct Measurement of Oleocanthal and Oleacein Levels in Olive Oil by Quantitative 1H NMR. Establishment of a New Index for the Characterization of Extra Virgin Olive Oils: E. Karkoula, et al.; J. Agric. Food Chem. 60, 11696 (2012) | Inhibition of human neutrophils NEP activity, CD11b/CD18 expression and elastase release by 3,4-dihydroxy-phenylethanol-elenolic acid dialdehyde, oleacein: M.E. Czerwinska, et al.; Food Chem. 153, 1 (2014) | One-step semisynthesis of oleacein and the determination as a 5-lipoxygenase inhibitor: K. Vougogiannopoulou, et al.; J. Nat. Prod. 77, 441 (2014) | Oleacein. translation from Mediterranean diet to potential antiatherosclerotic drug: M. Naruszewicz, et al.; Curr. Pharm. Des. 21, 1205 (2015) (Review) | Effects of Oleacein on High-Fat Diet-Dependent Steatosis, Weight Gain, and Insulin Resistance in Mice: G.E. Lombardo, et al.; Front. Endocrinol. 9, 116 (2018) | The extra virgin olive oil phenolic oleacein is a dual substrate-inhibitor of catechol-O-methyltransferase: E. Cuyas, et al.; Food Chem. Toxicol. 128, 35 (2019) | Computational de-orphanization of the olive oil biophenol oleacein: Discovery of new metabolic and epigenetic targets: E. Cuyas, et al.; Food Chem. Toxicol. 131, 110529 (2019) | Anti-tumor Activity and Epigenetic Impact of the Polyphenol Oleacein in Multiple Myeloma: G. Juli, et al.; Cancers 11, E990 (2019) | Oleacein Prevents High Fat Diet-Induced Adiposity and Ameliorates Some Biochemical Parameters of Insulin Sensitivity in Mice: S.M. Lepore, et al.; Nutrients 11, E1829 (2019) | The Extra-Virgin Olive Oil Polyphenols Oleocanthal and Oleacein Counteract Inflammation-Related Gene and miRNA Expression in Adipocytes by Attenuating NF-kappaB Activation: S. Carpi, et al.; Nutrients 11, E2855 (2019) | Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health: A. Karkovic Markovic, et al.; Molecules 24, E2001 (2019) | Oleacein inhibits STAT3, activates the apoptotic machinery, and exerts anti-metastatic effects in the SH-SY5Y human neuroblastoma cells: S. Cirmi, et al. Food Function 11, 3271 (2020)