Silymarin
| Code | Size | Price |
|---|
| CDX-S0289-G025 | 25 g | £65.00 |
Quantity:
| CDX-S0289-G050 | 50 g | £108.00 |
Quantity:
| CDX-S0289-G100 | 100 g | £164.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
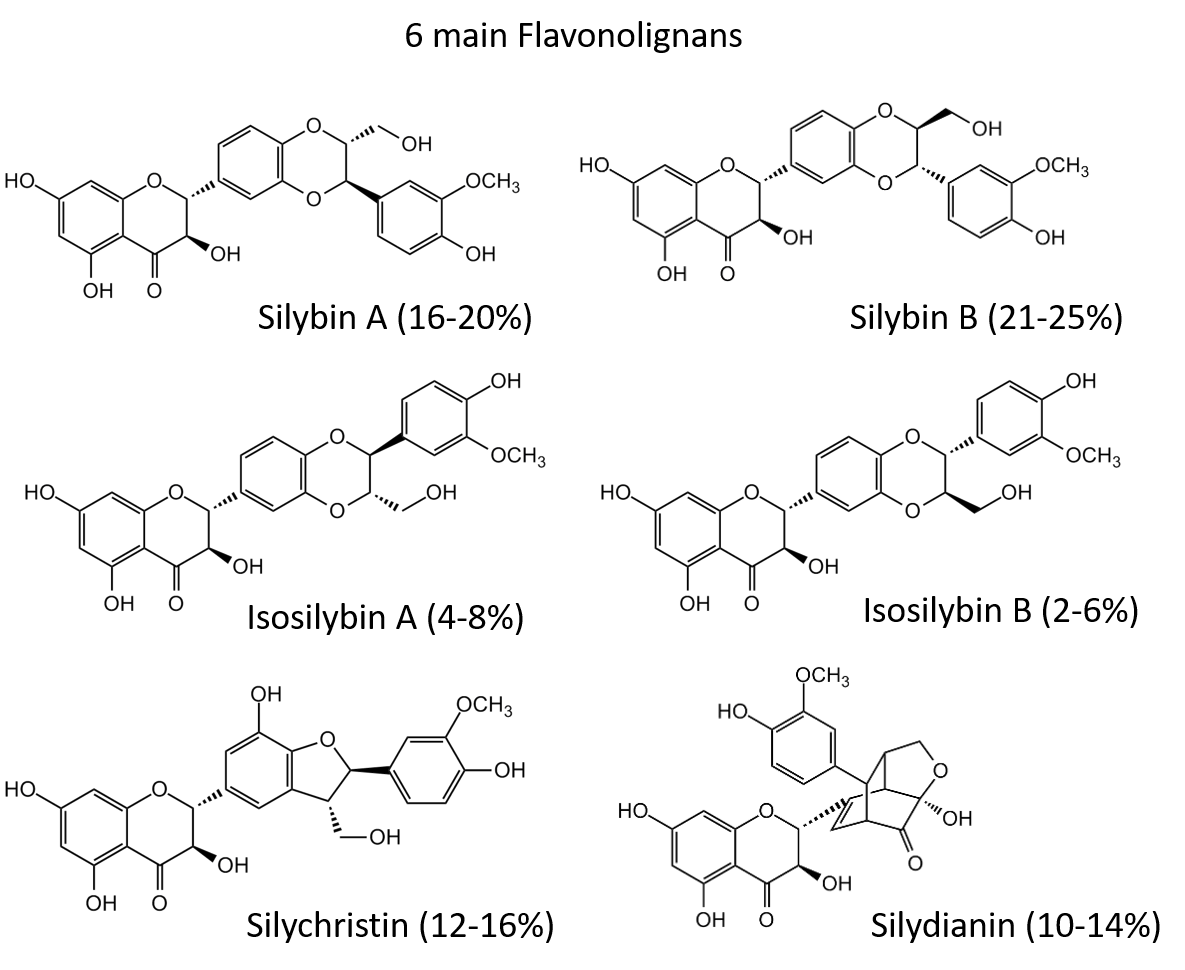
Mixture of flavanonolderivates Silibinin, Isosilibinin, Silicristin and Silidianin.
Appearance:
Yellow to brown powder.
CAS:
65666-07-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H315, H319, H335
Long Description:
Chemical mixture. Silymarin is a polyphenolic flavonoid extracted from the seeds of Silybum marianum or milk thistle. Silymarin is an isomeric mixture of the flavonolignans silydianin, silychristin, silibinin [Silybin] and isosilybin. It has similar biological properties to its main component Silibinin, including potent antioxidant, anti-inflammatory, anticancer, antidiabetic, hepatoprotective, neuroprotective, cardioprotective, antiviral, antimicrobial and immunosuppressive activities.
MDL:
MFCD01776359
Package Type:
Vial
Precautions:
P264, P280, P362+364, P261
Product Description:
Silymarin is a polyphenolic flavonoid extracted from the seeds of Silybum marianum or milk thistle. Silymarin is an isomeric mixture of the flavonolignans silydianin, silychristin, silibinin [Silybin] and isosilybin. It has similar biological properties to its main component Silibinin, including potent antioxidant, anti-inflammatory, anticancer, antidiabetic, hepatoprotective, neuroprotective, cardioprotective, antiviral, antimicrobial and immunosuppressive activities.
Purity:
>30% (HPLC Silybin)
Signal word:
Warning
Solubility Chemicals:
Soluble in DMSO or ethanol (1mg/ml).
Source / Host:
Plant
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) N. Skottova & V. Krecman; Physiol. Res. 47, 1 (1998) (Review) | (2) R.P. Singh & R. Agarwal; Antioxid. Redox Signal. 4, 655 (2002) (Review) | (3) S.K. Katiyar; Int. J. Oncol. 26, 169 (2005) (Review) | (4) K.E. Mayer, et al.; J. Viral. Hepat. 12, 559 (2005) (Review) | (5) R. Agarwal, et al.; Anticancer Res. 26, 4457 (2006) (Review) | (6) K. Ramasamy & R. Agarwal; Cancer Lett. 269, 352 (2008) (Review) | (7) M. Rafieian-Kopaie & H. Nasri; J. Renal Inj. Prev. 1, 3 (2012) (Review) | (8) S.J. Polyak, et al.; Hepatology 57, 1262 (2013) (Review) | (9) A. Borah, et al.; CNS Neurosci. Ther. 19, 847 (2013) (Review) | (10) P.F. Surai; Antioxidants 4, 204 (2015) (Review) | (11) L. Voroneanu, et al.; J. Diabetes Res. 2016, 5147468 (2016) (Review) | (12) K.P. Devi, et al.; Curr. Drug Targets 18, 1529 (2017) (Review) | (13) N. Esmaeil, et al.; Int. Immunopharmacol. 50, 194 (2017) (Review) | (14) M. Vahabzadeh, et al.; J. Sci. Food Agric. 98, 4816 (2018) (Review) | (15) T. Hosseinabadi, et al.; Phytother. Res. 33, 2849 (2019) (Review)