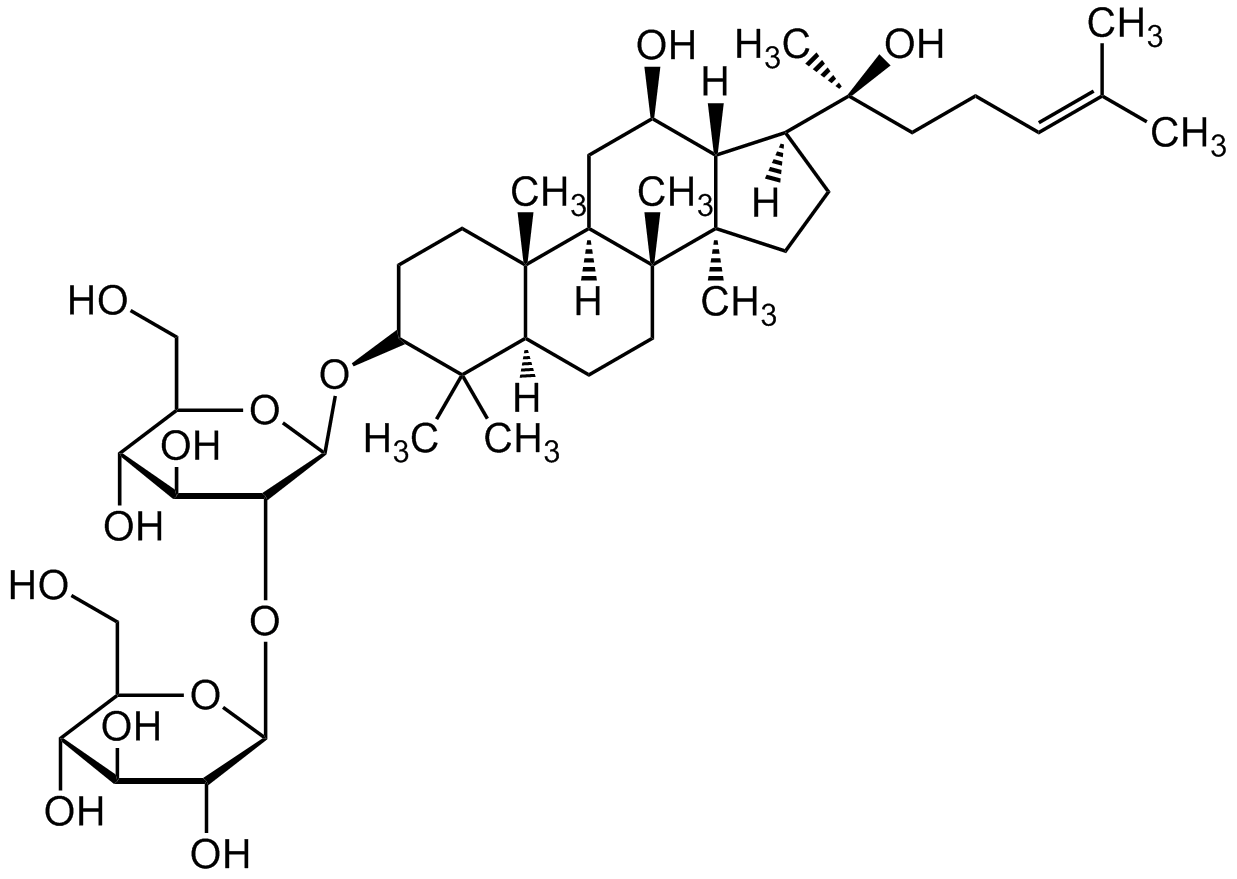
Ginsenoside Rg3
| Code | Size | Price |
|---|
| CDX-G0220-M005 | 5 mg | £35.00 |
Quantity:
| CDX-G0220-M025 | 25 mg | £84.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +20°C, Long Term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
20(S)-Ginsenoside-Rg3; Rg3; (3beta,12beta)-12,20-Dihydroxydammar-24-en-3-yl 2-O-beta-D-glucopyranosyl-beta-D-glucopyranoside
Appearance:
White to beige powder.
CAS:
14197-60-5
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C42H72O13/c1-21(2)10-9-14-42(8,51)22-11-16-41(7)29(22)23(45)18-27-39(5)15-13-28(38(3,4)26(39)12-17-40(27,41)6)54-37-35(33(49)31(47)25(20-44)53-37)55-36-34(50)32(48)30(46)24(19-43)52-36/h10,22-37,43-51H,9,11-20H2,1-8H3/t22-,23+,24+,25+,26-,27+,28+,29-,30+,31+,32-,33-,34+,35+,36-,37-,39-,40+,41+,42-/m0/s1
InChiKey:
RWXIFXNRCLMQCD-XRBUSOSMSA-N
Long Description:
Chemical. CAS: 14197-60-5. Formula: C42H72O13. MW: 785.01. Ginsenoside Rg3 is a panaxadiol found in white and red P. ginseng. Show a broad range of biological in vitro and in vivo effects, including anticancer, antidiabetic, neuroprotective, antioxidant, anti-hypertensive, and anti-inflammatory actions. The anticancer mechanisms include induction of apoptosis and autophagy, inhibition of proliferation, inhibition of metastasis and angiogenesis, cell cycle arrest, immunomodulatory effects, sensitization to radiation, reducing multidrug resistance and inducing genotoxicity to the cancer cells. Ginsenoside Rg3 has been shown to inhibit the 5-HT3A and alpha3beta4 nACh receptors, the voltage-dependent Ca2+, K+, and Na+ channel currents. It is a scavenger of hydroxyl radicals and downregulated the expression of DNA methyltransferases, reducing global DNA methylation, modifying the methylation of the promoter region of some relevant genes in cancer. It enhances glucose-stimulated insulin secretion and activates AMPK. Ginsenoside Rg3 regulates NF-kappaB activity and suppresses the NLRP3 inflammasome activation through inhibition of its assembly.
MDL:
MFCD06410950
Molecular Formula:
C42H72O13
Molecular Weight:
785.01
Package Type:
Vial
Product Description:
Ginsenoside Rg3 is a panaxadiol found in white and red P. ginseng. Show a broad range of biological in vitro and in vivo effects, including anticancer, antidiabetic, neuroprotective, antioxidant, anti-hypertensive, and anti-inflammatory actions. The anticancer mechanisms include induction of apoptosis and autophagy, inhibition of proliferation, inhibition of metastasis and angiogenesis, cell cycle arrest, immunomodulatory effects, sensitization to radiation, reducing multidrug resistance and inducing genotoxicity to the cancer cells. Ginsenoside Rg3 has been shown to inhibit the 5-HT3A and alpha3beta4 nACh receptors, the voltage-dependent Ca2+, K+, and Na+ channel currents. It is a scavenger of hydroxyl radicals and downregulated the expression of DNA methyltransferases, reducing global DNA methylation, modifying the methylation of the promoter region of some relevant genes in cancer. It enhances glucose-stimulated insulin secretion and activates AMPK. Ginsenoside Rg3 regulates NF-kappaB activity and suppresses the NLRP3 inflammasome activation through inhibition of its assembly.
Purity:
>98% (HPLC)
SMILES:
OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2O[C@H]3C(C)(C)[C@@](CC[C@]4(C)[C@]5([H])C[C@@H](O)[C@@]6([H])[C@]4(CC[C@@]6([C@@](C)(O)CC/C=C(C)/C)[H])C)([H])[C@]5(C)CC3)O1
Solubility Chemicals:
Soluble in DMSO (10mg/ml), DMF (10mg/ml) or ethanol (15mg/ml).
Source / Host:
Plant
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) M. Mochizuki, et al.; Biol. Pharm. Bull. 18, 1197 (1995) | (2) N.D. Kim, et al.; Eur. J. Pharmacol. 367, 41 (1999) | (3) H. Rhim, et al.; Eur. J. Pharmacol. 436, 151 (2002) | (4) S. Kim, et al.; BBRC 323, 416 (2004) | (5) Q. Zhang, et al.; BBRC 342, 824 (2006) | (6) M.W. Park, et al.; Biol. Pharm. Bull. 31, 748 (2008) | (7) J.T. Hwang, et al.; Phytother. Res. 23, 262 (2009) | (8) J. Tian, et al.; Phytother. Res. 23, 486 (2009) | (9) X. Wei, et al.; Fitoterapia 83, 636 (2012) | (10) Y.M. Shin, et al.; Mol. Biol. Rep. 40, 269 (2013) | (11) D.G. Kim, et al.; Oncotarget 5, 4438 (2014) | (12) K.J. Son, et al.; Immune Netw. 16, 75 (2016) | (13) H.Y. Sun, et al.; Anticancer Res. 36, 4647 (2016) | (14) M. Sun, et al.; Int. J. Mol. Med. 39, 507 (2017) (Review) | (15) I.S. Lee, et al.; J. Immunol. Res. 2016, 7521601 (2016) | (16) L. Zhang, et al.; Front. Pharmacol. 8, 113 (2017) | (17) M. Nakhjavani, et al.; Medicines 6, E17 (2019) (Review) | (18) Y. Shi, et al.; FASEB J. 34, 208 (2020)