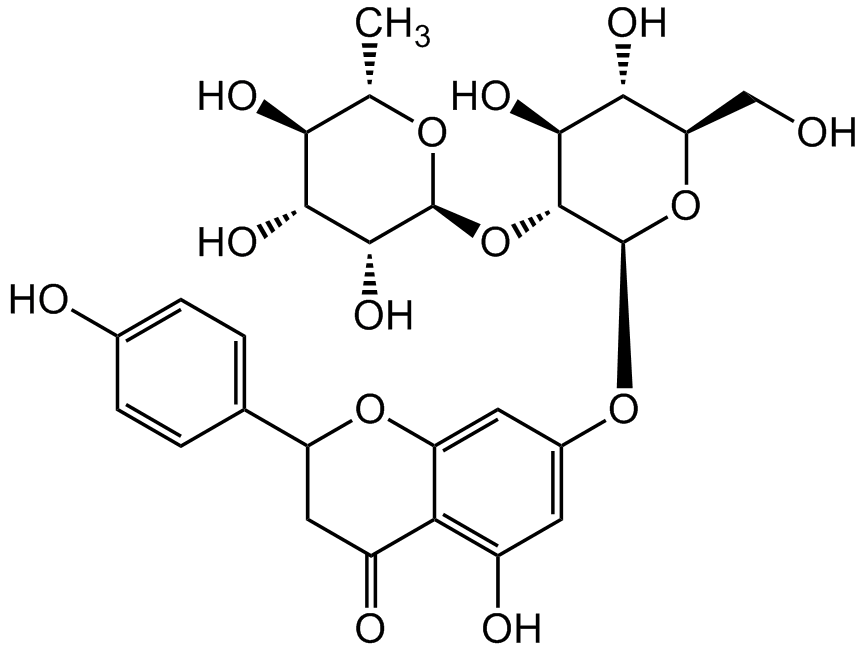
Naringin
| Code | Size | Price |
|---|
| CDX-N0234-G100 | 100 g | £108.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +20°C Long Term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
4',5,7-Trihydroxyflavanone 7-rhamnoglucoside; Naringenin 7-neohesperidoside; Naringenine-7-rhamnosidoglucoside; Naringoside; NSC 5548
Appearance:
Light yellow to beige powder.
CAS:
10236-47-2
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H315-H319-H335
InChi:
InChI=1S/C27H32O14/c1-10-20(32)22(34)24(36)26(37-10)41-25-23(35)21(33)18(9-28)40-27(25)38-13-6-14(30)19-15(31)8-16(39-17(19)7-13)11-2-4-12(29)5-3-11/h2-7,10,16,18,20-30,32-36H,8-9H2,1H3/t10-,16?,18+,20-,21+,22+,23-,24+,25+,26-,27+/m0/s1
InChiKey:
DFPMSGMNTNDNHN-JJLSSNRUSA-N
Long Description:
Chemical. CAS: 10236-47-2. Formula: C27H32O14. MW: 580.54. Naringin is a citrus-derived flavonoid (bitter principal). It undergoes extensive metabolism in the liver. It has a broad panel of properties, including anti-inflammatory, antioxidant, anticancer, antidiabetic, cardioprotective, bone regenerative and neuroprotective activities. Naringin acts by modulating several signaling pathway targets, activity is primarily attributed to its anti-inflammatory (via inhibiting recruitment of cytokines and inflammatory transcription factors) and antioxidant (via scavenging of free radicals, bolstering of endogenous antioxidant defense system and metal ion chelation) effects. It increases the expression of neurotrophic factor in dopaminergic neurons, providing neuroprotection.
MDL:
MFCD00148888
Molecular Formula:
C27H32O14
Molecular Weight:
580.54
Package Type:
Vial
Precautions:
P261-P305 + P351 + P338
Product Description:
Naringin is a citrus-derived flavonoid (bitter principal). It undergoes extensive metabolism in the liver. It has a broad panel of properties, including anti-inflammatory, antioxidant, anticancer, antidiabetic, cardioprotective, bone regenerative and neuroprotective activities. Naringin acts by modulating several signaling pathway targets, activity is primarily attributed to its anti-inflammatory (via inhibiting recruitment of cytokines and inflammatory transcription factors) and antioxidant (via scavenging of free radicals, bolstering of endogenous antioxidant defense system and metal ion chelation) effects. It increases the expression of neurotrophic factor in dopaminergic neurons, providing neuroprotection.
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
OC1=C2C(OC(C3=CC=C(O)C=C3)CC2=O)=CC(O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O[C@@H]5O[C@@H](C)[C@H](O)[C@@H](O)[C@H]5O)=C1
Solubility Chemicals:
Soluble in DMSO (10mg/ml), DMF (20mg/ml) or ethanol (1mg/ml).
Source / Host:
Plant
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) U. Fuhr, et al.; Br. J. Clin. Pharmacol. 35, 431 (1993) | (2) P.C. Ho & D.J. Saville; J. Pharm. Pharmaceut. Sci. 4, 217 (2001) | (3) S. Bharti, et al.; Planta Med. 80, 437 (2014) (Review) | (4) U.J. Jung, et al.; Exp. Neurobiol. 23, 124 (2014) (Review) | (5) M.A. Alam, et al.; Adv. Nutr. 5, 404 (2014) (Review) | (6) R. Chen, et al.; Pharm. Biol. 54, 3203 (2016) (Review) | Anti-estrogenic and anti-aromatase activities of citrus peels major compounds in breast cancer: D.M. El-Kersh, et al.; Nature Sci. Rep. 11, 7121 (2021)