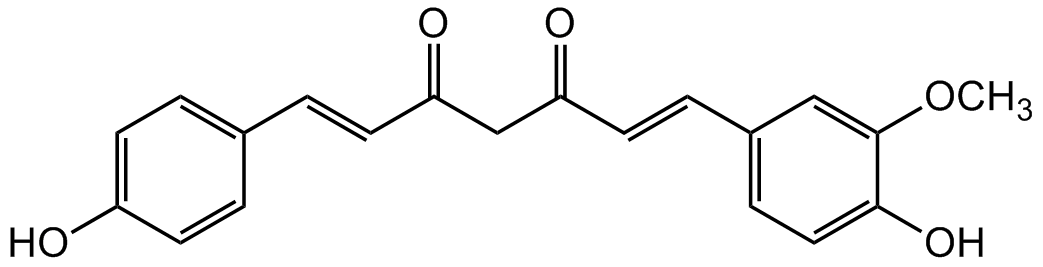
Demethoxycurcumin
| Code | Size | Price |
|---|
| CDX-D0929-M005 | 5 mg | £89.00 |
Quantity:
| CDX-D0929-M025 | 25 mg | £280.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +20°C, Long Term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
DMC; (E,E)-1-(4-Hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione; Desmethoxycurcumin; Monodemethoxycurcumin
Appearance:
Yellow to orange powder.
CAS:
22608-11-3
Class:
9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS09
Handling Advice:
Protect from light and moisture.
Hazards:
H400
InChi:
InChI=1S/C20H18O5/c1-25-20-12-15(6-11-19(20)24)5-10-18(23)13-17(22)9-4-14-2-7-16(21)8-3-14/h2-12,21,24H,13H2,1H3/b9-4+,10-5+
InChiKey:
HJTVQHVGMGKONQ-LUZURFALSA-N
Long Description:
Chemical. CAS: 22608-11-3. Formula: C20H18O5. MW: 338.35. Demethoxycurcumin (DMC) is a natural demethoxy derivative of curcumin with anti-inflammatory and anti-cancer properties. DMC suppresses cell proliferation, migration and invasion in cancer cells. It down-regulates the transcriptional coactivator p300, suppressing the Wnt/beta-catenin pathway, and inhibits lipopolysaccharide induction of iNOS by blocking NF-kappa activation. It also has been shown to inhibit energy metabolic and oncogenic signaling pathways through AMPK activation. In addition it is a potent inhibitor of P-Type ATPases. Shown to have neuroprotective properties. It also has anti-plasmodial activity.
MDL:
MFCD03427310
Molecular Formula:
C20H18O5
Molecular Weight:
338.35
Package Type:
Vial
PG:
III
Precautions:
P273
Product Description:
Demethoxycurcumin (DMC) is a natural demethoxy derivative of curcumin with anti-inflammatory and anti-cancer properties. DMC suppresses cell proliferation, migration and invasion in cancer cells. It down-regulates the transcriptional coactivator p300, suppressing the Wnt/beta-catenin pathway, and inhibits lipopolysaccharide induction of iNOS by blocking NF-kappa activation. It also has been shown to inhibit energy metabolic and oncogenic signaling pathways through AMPK activation. In addition it is a potent inhibitor of P-Type ATPases. Shown to have neuroprotective properties. It also has anti-plasmodial activity.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
OC1=CC=C(/C=C/C(CC(/C=C/C2=CC=C(O)C(OC)=C2)=O)=O)C=C1
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or DMF (10mg/ml).
Source / Host:
Plant
Transportation:
Non-hazardous
UN Nummer:
3077
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) S.K. Sandur, et al.; Carcinogenesis 28, 1765 (2007) | (2) L.Y. Guo, et al.; Arch. Pharm. Res. 31, 490 (2008) | (3) M.J. Ryu, et al.; BBRC 377, 1304 (2008) | (4) L. Zhang, et al.; Int. Immunopharmacol. 10, 331 (2010) | (5) Y.L. Liu, et al.; Mol. Med. Rep. 4, 675 (2011) | (6) X. Ni, et al.; Oncol. Rep. 28, 85 (2012) | (7) O.B. Villaflores, et al.; Taiwan. J. Obstet. Gynecol. 51, 554 (2012) | (8) J.M. Shieh, et al.; J. Agric. Food Chem. 61, 6366 (2013) | (9) R. Munigunti, et al.; Nat. Prod. Res. 28, 359 (2014) | (10) T.T. Dao, et al.; PLoS One 11, e0163260 (2016) | (11) M. Ramkumar, et al.; BMC Complement Altern. Med. 17, 217 (2017) | (12) C.C. Lin, et al.; Anticancer Res. 38, 2761 (2018) | (13) M. Hatamipour, et al.; J. Cell Physiol. 233, 9247 (2018) | (14) M. Hatamipour, et al.; J. Cell Physiol. 234, 19320 (2019)