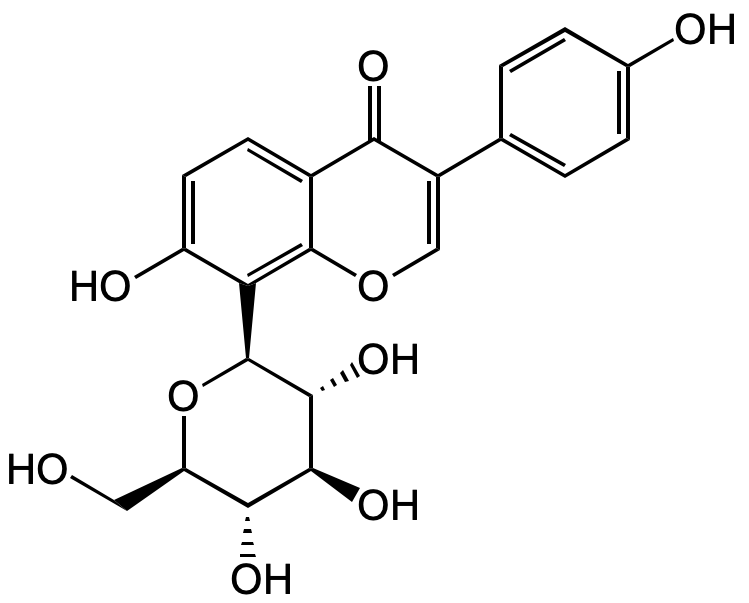
Puerarin
| Code | Size | Price |
|---|
| CDX-P0250-M250 | 250 mg | £169.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +20°C Long Term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Kakonein; NPI 031G; 8-(beta-D-Glucopyranosyl-7-hydroxy-3- (4-hydroxyphenyl)-4H-1-benzopyran-4-one
Appearance:
White to off white powder.
CAS:
3681-99-0
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C21H20O9/c22-7-14-17(26)18(27)19(28)21(30-14)15-13(24)6-5-11-16(25)12(8-29-20(11)15)9-1-3-10(23)4-2-9/h1-6,8,14,17-19,21-24,26-28H,7H2/t14-,17-,18+,19-,21+/m1/s1
InChiKey:
HKEAFJYKMMKDOR-VPRICQMDSA-N
Long Description:
Chemical. CAS: 3681-99-0. Formula: C21H20O9. MW: 416.38. Isolated from plant source. Puerarin is a natural isoflavone isolated from plants of the genus Pueraria used in traditional Chinese herbal medicine. Daidzein and glucuronides are the main metabolites of puerarin. It has been widely used in the treatment of cardiovascular and cerebrovascular diseases, diabetes and diabetic complications, osteonecrosis, Parkinson's disease, Alzheimer's disease, endometriosis, and cancer. The beneficial effects of puerarin on the various medicinal purposes may be due to its wide spectrum of pharmacological properties such as vasodilation, cardioprotection, neuroprotection, antioxidant, anticancer, anti-inflammation, alleviating pain, promoting bone formation, inhibiting alcohol intake and attenuating insulin resistance. Due to the poor water-solubility and liposolubility, the applications of puerarin are limited. Strategies to improve the bioavailability of puerarin are ongoing, various nanotechnologies and preparation technologies including microemulsions and SMEDDS, dendrimers, nanoparticles and nanocrystals have been researched to improve the bioavailability of puerarin.
MDL:
MFCD00076007
Molecular Formula:
C21H20O9
Molecular Weight:
416.38
Package Type:
Vial
Product Description:
Puerarin is a natural isoflavone isolated from plants of the genus Pueraria used in traditional Chinese herbal medicine. Daidzein and glucuronides are the main metabolites of puerarin. It has been widely used in the treatment of cardiovascular and cerebrovascular diseases, diabetes and diabetic complications, osteonecrosis, Parkinson's disease, Alzheimer's disease, endometriosis, and cancer. The beneficial effects of puerarin on the various medicinal purposes may be due to its wide spectrum of pharmacological properties such as vasodilation, cardioprotection, neuroprotection, antioxidant, anticancer, anti-inflammation, alleviating pain, promoting bone formation, inhibiting alcohol intake and attenuating insulin resistance. Due to the poor water-solubility and liposolubility, the applications of puerarin are limited. Strategies to improve the bioavailability of puerarin are ongoing, various nanotechnologies and preparation technologies including microemulsions and SMEDDS, dendrimers, nanoparticles and nanocrystals have been researched to improve the bioavailability of puerarin.
Purity:
>98% (HPLC)
SMILES:
O=C1C(C2=CC=C(O)C=C2)=COC3=C([C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)C(O)=CC=C31
Solubility Chemicals:
Soluble in DMSO (10mg/ml), DMF (10mg/ml), ethanol (5mg/ml) or methanol (1mg/ml).
Source / Host:
Isolated from plant source.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) M.K. Choo, et al.; Biol. Pharm. Bull. 25, 1328 (2002) | (2) D.H. Overstreet, et al.; Pharm. Biochem. Beh. 75, 619 (2003) | (3) F.S. Chueh, et al.; J. Pharmacol. Sci. 96, 420 (2004) | (4) K. Nakamura, et al.; Chem. Pharm. Bull. 59, 23 (2011) | (5) S. Michihara, et al.; J. Nutr. Sci. Vitaminol. 58, 202 (2012) | (6) X.Y. Zhou, et al.; Phytother. Res. 28, 961 (2014) | (7) H.W. Jung, et al.; Nutrients 8, E33 (2017) | (8) H. Hongyun, et al.; Neurosci. Lett. 643, 45 (2017) | (9) M.Z. Int. Immunopharmacol. 61, 306 (2018) | (10) L. Zhang; Drug. Deliv. 26, 860 (2019) | (11) Y. Yi, et al.; J. Mol. Model 25, 202 (2019) | (12) D. Lian, et al.; Phytomedicine 55, 310 (2019) | (13) L. Jia, et al.; Exp. Ther. Med. 18, 543 (2019) | (14) G. Ye, et al.; Oncol. Lett. 17, 195 (2019) | (15) T. Liang, et al.; Mol. Biol. Rep. (Epub ahead of print) (2019)