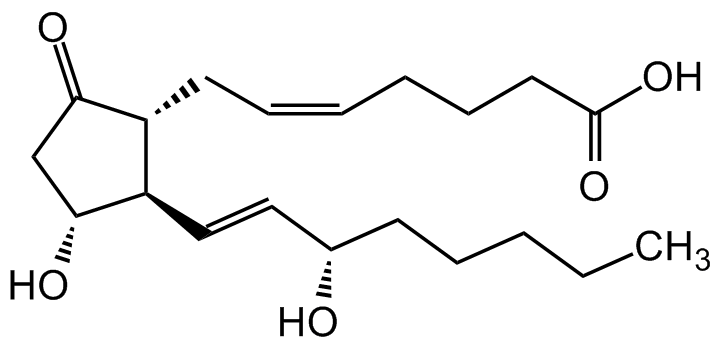
Prostaglandin E2
| Code | Size | Price |
|---|
| CDX-P0318-M005 | 5 mg | £84.00 |
Quantity:
| CDX-P0318-M010 | 10 mg | £121.00 |
Quantity:
| CDX-P0318-M025 | 25 mg | £230.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +4°C, Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Dinoprostone; ((5Z,11a,13E,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dienoic acid; PGE2
Appearance:
White to light yellow solid.
CAS:
363-24-6
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H302-H360
InChi:
InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1
InChiKey:
XEYBRNLFEZDVAW-ARSRFYASSA-N
Long Description:
Chemical. CAS: 363-24-6. Formula: C20H32O5. MW: 352.47. Prostaglandin E2 is one of the most typical lipid mediators produced from arachidonic acid (AA) by cyclooxygenase (COX) as the rate-limiting enzyme and acts on four kinds of receptor subtypes (EP1, EP2, EP3 and EP4) with high binding affinity. It is a regulator of numerous physiological functions ranging from reproduction to neuronal, metabolic and immune functions. It acts as a pro-inflammatory mediator, but also exerts anti-inflammatory responses. As pro-inflammatory mediator, PGE2 contributes to the regulation of the cytokine expression profile of DCs and has been reported to bias T cell differentiation towards a T helper (Th) 1 or Th2 response. It exerts anti-inflammatory actions on innate immune cells like neutrophils, monocytes and NK cells. Prostaglandin E2 also facilitates tumor progression through stimulation of angiogenesis via EP2, mediates cell invasion and metastasis formation via EP4 and promotes cell survival by inhibiting apoptosis via numerous signaling pathways. Recently it has been shown to inhibit NLRP3 by acting through receptor EP4 activation that increases cAMP. cAMP activates PKA that phosphorylates NLRP3 at serine 295. PKA phosphorylation of NLRP3 inhibits the NLRP3 ATPase activity, which is required for assembly of NLRP3-ASC complexes.
MDL:
MFCD00077861
Molecular Formula:
C20H32O5
Molecular Weight:
352.47
Package Type:
Vial
Precautions:
P201-P308 + P313
Product Description:
Prostaglandin E2 is one of the most typical lipid mediators produced from arachidonic acid (AA) by cyclooxygenase (COX) as the rate-limiting enzyme and acts on four kinds of receptor subtypes (EP1, EP2, EP3 and EP4) with high binding affinity. It is a regulator of numerous physiological functions ranging from reproduction to neuronal, metabolic and immune functions. It acts as a pro-inflammatory mediator, but also exerts anti-inflammatory responses. As pro-inflammatory mediator, PGE2 contributes to the regulation of the cytokine expression profile of DCs and has been reported to bias T cell differentiation towards a T helper (Th) 1 or Th2 response. It exerts anti-inflammatory actions on innate immune cells like neutrophils, monocytes and NK cells. Prostaglandin E2 also facilitates tumor progression through stimulation of angiogenesis via EP2, mediates cell invasion and metastasis formation via EP4 and promotes cell survival by inhibiting apoptosis via numerous signaling pathways. Recently it has been shown to inhibit NLRP3 by acting through receptor EP4 activation that increases cAMP. cAMP activates PKA that phosphorylates NLRP3 at serine 295. PKA phosphorylation of NLRP3 inhibits the NLRP3 ATPase activity, which is required for assembly of NLRP3-ASC complexes.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
O=C1C[C@@H](O)[C@H](/C=C/[C@@H](O)CCCCC)[C@H]1C/C=CCCCC(O)=O
Solubility Chemicals:
Soluble in ethanol (50 mg/ml), DMSO (50 mg/ml) or DMF (100 mg/ml). Sparingly soluble in PBS, pH 7.2 (5mg/ml).
Source / Host:
Microbial
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) D. F. Legler, et al.; Int. J. Biochem. Cell Biol. 42, 198 (2010) (Review) | (2) D. Sakata, et al.; J. Pharmacol. Sci. 112, 1 (2010) (Review) | (3) E.M. Durand, et al.; Curr. Opin. Hematol. 17, 308 (2010) (Review) | (4) J. Reader, et al.; Cancer Metastasis Rev. 30, 449 (2011) (Review) | (5) V. Sreeramkumar, et al.; Immunol. Cell Biol. 90, 579 (2012) (Review) | (6) P. Kalinski; J. Immunol. 188, 21 (2012) (Review) | (7) M. Rodriguez, et al.; Mol. Pharmacol. 85, 187 (2014) (Review) | (8) K. Kawahara, et al.; Biochim. Biophys Acta. 1851, 414 (2015) (Review) | (9) M. Sokolowska, et al.; J. Immunol. 194, 5472 (2015)