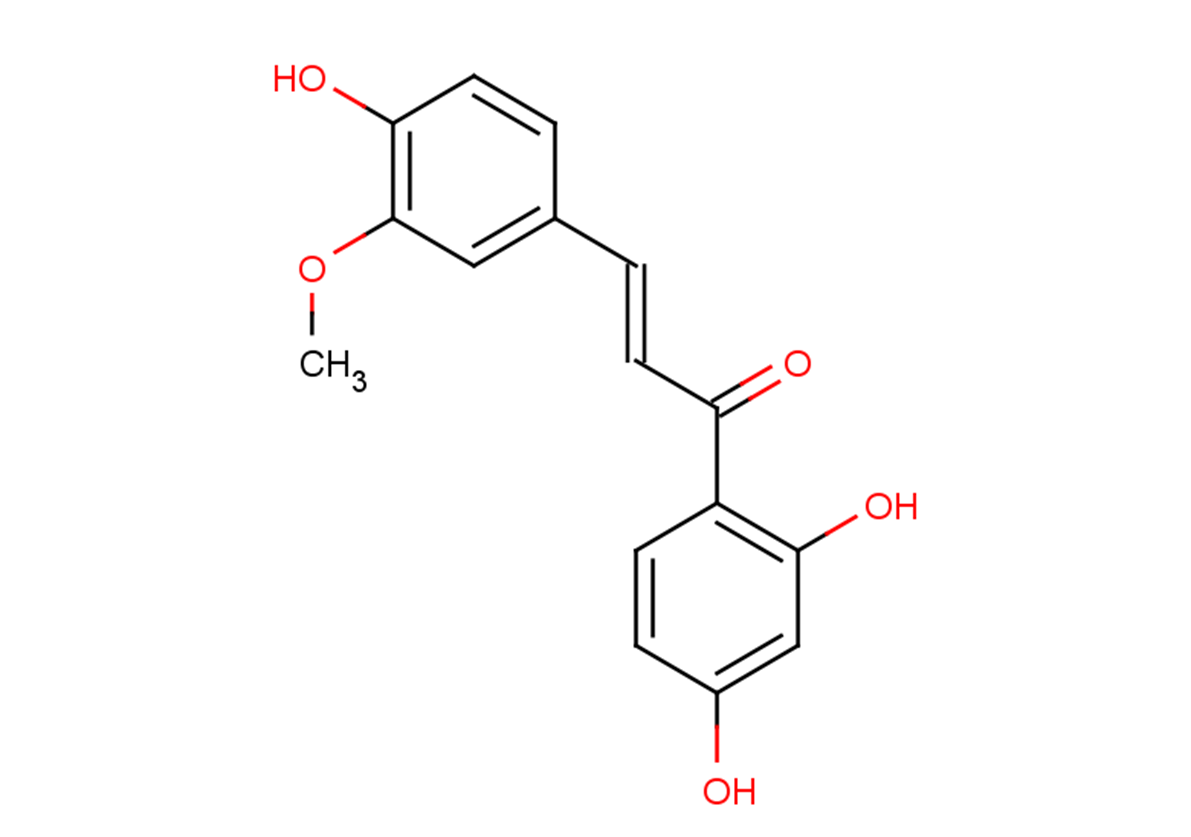
Homobutein
| Code | Size | Price |
|---|
| TAR-TN4220-1mg | 1mg | £227.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-TN4220-2mg | 2mg | £306.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-TN4220-5mg | 5mg | £430.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-TN4220-10mg | 10mg | £600.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-TN4220-25mg | 25mg | £863.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-TN4220-50mg | 50mg | £1,179.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
cool pack
Storage:
-20℃
Images
Documents
Further Information
Bioactivity:
Homobutein is a dual inhibitor of HDACs and NF-κB with IC50s of 190 and 38 μM. Homobutein is an iron chelator with anticancer, anti-inflammatory, antiparasite, and antioxidation activities.
CAS:
34000-39-0
Formula:
C16H14O5
Molecular Weight:
286.28
Pathway:
DNA Damage/DNA Repair; oxidation-reduction; Immunology/Inflammation; Microbiology/Virology; Chromatin/Epigenetic; NF-κb
Purity:
0.995
SMILES:
C(=C/C(=O)C1=C(O)C=C(O)C=C1)C2=CC(OC)=C(O)C=C2
Target:
Antioxidant; NF-κB; HDAC; Parasite; Immunology/Inflammation related
References
Serobatse K, et al. Antioxidant and antimalarial properties of butein and homobutein based on their ability to chelate iron (II and III) cations: a DFT study in vacuo and in solution. European Food Research and Technology, 2016, 242(1): 71-90.
Orlikova B, et al. Natural chalcones as dual inhibitors of HDACs and NF-?B. Oncol Rep. 2012 Sep;28(3):797-805.
Adeyemi OS, et al. In Vitro Screening to Identify Anti-Toxoplasma Compounds and In Silico Modeling for Bioactivities and Toxicity. Yale J Biol Med. 2019 Sep 20;92(3):369-383.
Yenesew A, et al. Anti-plasmodial flavonoids from the stem bark of Erythrina abyssinica. Phytochemistry. 2004 Nov;65(22):3029-32.