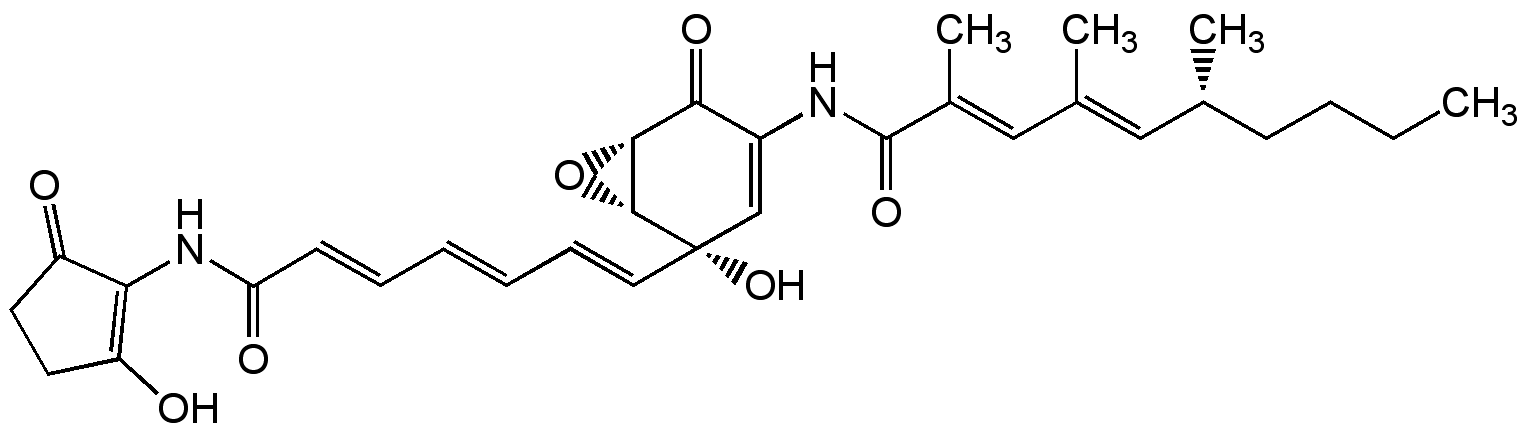
Manumycin A
Product Code: AG-CN2-2000
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-2000-M001 | 1 mg | £110.00 |
Quantity:
| AG-CN2-2000-M005 | 5 mg | £410.00 |
Quantity:
| AG-CN2-2000-M010 | 10 mg | £610.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
BLUE ICE
Storage:
Short Term Storage: +4°C. Long Term Storage: -20°C
Images
Further Information
Appearance:
Yellow to brown powder.
CAS:
52665-74-4
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light.
Hazards:
H302, H312, H319
InChi:
InChI=1S/C31H38N2O7/c1-5-6-11-19(2)16-20(3)17-21(4)30(38)32-22-18-31(39,29-28(40-29)27(22)37)15-10-8-7-9-12-25(36)33-26-23(34)13-14-24(26)35/h7-10,12,15-19,28-29,34,39H,5-6,11,13-14H2,1-4H3,(H,32,38)(H,33,36)/b8-7+,12-9+,15-10+,20-16+,21-17+/t19-,28-,29-,31+/m1/s1
InChiKey:
TWWQHCKLTXDWBD-MVTGTTCWSA-N
Long Description:
Chemical. CAS: 52665-74-4. Formula: C31H38N2O7. MW: 550.6. Antibiotic. Apoptosis and endoplasmic reticulum stress-mediated cell death inducer. Potent, selective and competitive cell permeable rasfarnesyltransferase inhibitor (IC50 = 30 nM). Does not affect geranylgeranyltransferase (IC50 = 180 uM). Inhibition is competitive with respect to farnesyl pyrophosphate and non-competitive with respect to Ras. Neutral sphingomyelinase inhibitor. Blocks insulin-induced MAP kinase activation in rat cardiac myocytes (19 uM). Targets protein phosphatase 1alpha (PP1alpha) and reduces hydrogen peroxide. Corrects aberrant splicing of the muscle chloride channel Clcn1 in myotonic dystrophy type 1 (DM1). Shows potential anti-inflammatory activity. Irreversible human thioredoxin reductase 1 (TrxR-1) inhibitor (IC50=272nM) and inducer of NADPH oxidase activity. Acts very likely as a Michael acceptor to the nucleophilic Sec residue in the C-terminal redox center of TrxR-1 which yields a SecTRAP (selenium compromised thioredoxin reductase-derived apoptotic proteins), which promotes both apoptosis and necrosis via oxidative stress and increased intracellular reactive oxygen species (ROS) production. Due to the involvement of Ras during exosomes release, manumycin A has been investigated as an inhibitor of exosomes secretion. Acts against myotonic dystrophy type 1 (DM1). Mediates lymphoma apoptosis by targeting protein phosphatase 1alpha.
MDL:
MFCD18432704
Molecular Formula:
C3
Molecular Weight:
550.6
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Antibiotic. Apoptosis and endoplasmic reticulum stress-mediated cell death inducer. Potent, selective and competitive cell permeable rasfarnesyltransferase inhibitor (IC50 = 30 nM). Does not affect geranylgeranyltransferase (IC50 = 180 µM). Inhibition is competitive with respect to farnesyl pyrophosphate and non-competitive with respect to Ras. Neutral sphingomyelinase inhibitor. Blocks insulin-induced MAP kinase activation in rat cardiac myocytes (19 µM). Targets protein phosphatase 1alpha (PP1alpha) and reduces hydrogen peroxide. Corrects aberrant splicing of the muscle chloride channel Clcn1 in myotonic dystrophy type 1 (DM1). Shows potential anti-inflammatory activity. Irreversible human thioredoxin reductase 1 (TrxR-1) inhibitor (IC50=272nM) and inducer of NADPH oxidase activity. Acts very likely as a Michael acceptor to the nucleophilic Sec residue in the C-terminal redox center of TrxR-1 which yields a SecTRAP (selenium compromised thioredoxin reductase-derived apoptotic proteins), which promotes both apoptosis and necrosis via oxidative stress and increased intracellular reactive oxygen species (ROS) production. Due to the involvement of Ras during exosomes release, manumycin A has been investigated as an inhibitor of exosomes secretion. Acts against myotonic dystrophy type 1 (DM1). Mediates lymphoma apoptosis by targeting protein phosphatase 1alpha.
Product Line Areas NEW:
Antibiotic, Biochemicals, Natural Products, Cancer, Cell Death, Immunology, Metabolism
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
CCCC[C@@H](C)\C=C(/C)\C=C(/C)C(=O)NC1=C[C@@](O)(\C=C\C=C\C=C\C(=O)NC2=C(O)CCC2=O)[C@@H]2O[C@@H]2C1=O
Solubility Chemicals:
Soluble in DMSO or methanol. Insoluble in water.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Identification of Rasfarnesyltransferase inhibitors by microbial screening: M. Hara et al.; PNAS 90, 2281 (1993) | Inhibitors of Rasfarnesyltransferases: F. Tamanoi; TIBS 18, 349 (1993), (Review) | Farnesyltransferase inhibitors: Ras research yields a potential cancer therapeutic: J.B. Gibbs, et al.; Cell 77, 175 (1994) | Protein kinase C, but not tyrosine kinases or Ras, plays a critical role in angiotensin II-induced activation of Raf-1 kinase and extracellular signal-regulated protein kinases in cardiac myocytes: Y. Zou et al.; J. Biol. Chem. 271, 33592 (1996) | Manumycin inhibits ras signal transduction pathway and induces apoptosis in COLO320-DM human colon tumour cells: A. Di Paolo, et al.; Br. J. Cancer 82, 905 (2000) | Manumycin A and its analogues are irreversible inhibitors of neutral sphingomyelinase: C. Arenz, et al.; ChemBioChem 2, 141 (2001) | Enhancement of manumycin A-induced apoptosis by methoxyamine in myeloid leukemia cells: M. She, et al.; Leukemia 19, 595 (2005) | Binding of manumycin A inhibits IkappaB kinase beta activity: M. Bernier, et al.; J. Biol. Chem. 281, 2551 (2006) | A yeast-based genomic strategy highlights the cell protein networks altered by FTase inhibitor peptidomimetics: G. Porcu, et al.; Mol. Cancer 9:197 (2010) | Targeting farnesyl-transferase as a novel therapeutcstrategy for mevalonate kinase defiency: In vitro and in vivo approaches: L. De Leo, et al.; Pharmacol. Res. 61, 506 (2010) | Manumycin A corrects aberrant splicing of Clcn1 in myotonic dystrophy type 1 (DM1) mice: K. Oana, et al.; Sci. Rep. 3, 2142 (2013) | Manumycin induces apoptosis in prostate cancer cells: J.G. Li, et al. Onco Targets Ther. 7, 771 (2014) | The natural tumorcide Manumycin-A targets protein phosphatase 1alpha and reduces hydrogen peroxide to induce lymphoma apoptosis. G.B. Carey, et al.; Exp. Cell. Res. 332, 136 (2015) | Manumycin A from a new streptomyces strain induces endoplasmic reticulum stress-mediated cell death through specificity protein 1 signaling in human oral squamous cell carcinoma: C.J. Jae, et al.; Int. J. Oncol. 47, 1954 (2015) | Manumycin A downregulates release of proinflammatory cytokines from TNF alpha stimulated human monocytes: E. Cecrdlova, et al.; Immunol. Lett. 169, 8 (2016) | Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells: A. Datta, et al.; Cancer Lett. 408, 73 (2017) | Manumycin A Is a Potent Inhibitor of Mammalian Thioredoxin Reductase-1 (TrxR-1): A. Tuladhar & K.S. Rein; ACS Med. Chem. Lett. 9, 318 (2018) | The free fatty acid receptor 1 promotes airway smooth muscle cell proliferation through MEK/ERK and PI3K/Akt signaling pathways: A. Matoba, et al.; Am. J. Physiol. 314, 333 (2018) | Effectors of thioredoxin reductase: Brevetoxins and manumycin-A: A. Tuladhar, et al.; Comp. Biochem. Physiol. C Toxicol. Pharmacol. 217, 76 (2019) | Inhibiting extracellular vesicles formation and release: a review of EV inhibitors: M. Catalano & L. O'Driscoll; J. Etracell. Vesicles 9, 1703244 (2020) | Combination of photoactive hypericin and Manumycin A exerts multiple anticancer effects on oxaliplatin-resistant colorectal cells: M. Macejova, et al.; Toxicol. Vitro 66, 104860 (2020) | Manumycin polyketides act as molecular glues between UBR7 and P53: Y. Isobe, et al.; Nat. Chem. Biol. 16, 1189 (2020)