CycLex SIRT1/Sir2 Deacetylase Fluorometric Assay Kit Ver.2
| Code | Size | Price |
|---|
| MBL-CY-1151V2 | 100 Assays | £541.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Dry Ice
Storage:
-70°C
Images
Documents
Further Information
Background:
Sir2 is a conserved protein and was recently shown to regulate lifespan extension both in budding
yeast and nematode. In 2000, it was reported that the yeast Sir2 protein is a NAD(+)-dependent histone
deacetylase that plays a critical role in transcriptional silencing, genome stability and longevity. A human
homologue of Sir2, SIRT1, also functions as a NAD(+)-dependent-p53 deacetylase as well as a
NAD(+)-dependent histone deacetylase. SIRT1 was shown to regulate the activity of the p53 tumor
suppressor and inhibits apoptosis. These results have significant implications regarding an important role
for SIRT1 in modulating the sensitivity of cells in p53-dependent apoptotic response and the possible
effect in cancer therapy. Since the function of p53 is made to strengthen powerfully by using together
with DNA damaging reagent, it is expected that inhibitor of SIRT1 becomes an effective anticancer drug.
However, the conventional method for measuring SIRT1/Sir2 activity is very complicated and
laborious. In order to measure SIRT1/Sir2 enzyme activity, it is necessary to prepare radioactive
acetylated histone as a substrate. First, cells have to be labeled metabolically with radioactivity by
adding radioactive acetic acid to the culture medium. Second, radioactive acetylated histone has to be
purified from the cells. Following the reaction, it is necessary to extract and separate the radioactive
acetyl group, which has been released from acetylated histone, using ethyl acetate to measure the activity
of the enzyme based on the radioactivity.
Although a method for measuring the activity of deacetylase without the use of radioactive substances
was reported in recent years, owing to the use of fluorescent-labeled acetylated lysine as a substrate, the
reaction product must be separated from the intact substrate and the fluorescent intensity measured by
reverse phase HPLC. As mentioned above, these measurement systems are difficult to adapt for
processing many samples under a variety of conditions, because of their complicated operation. Thus a
simple system for biochemical analysis as well as for inhibitor screening without the use of radioactive
substances is preferred.
Description:
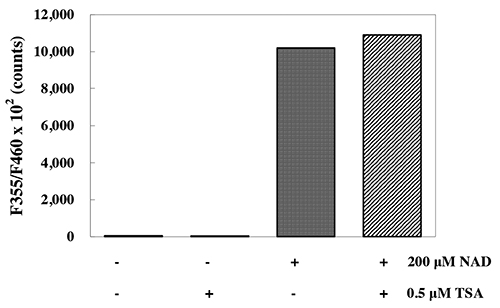
The CycLex Research Product CycLex SIRT1/Sir2 Deacetylase Fluorometric Assay kit detects
SIRT1/Sir2 activity in lysates. Primarily, the CycLex Research Product CycLex SIRT1/Sir2
Deacetylase Fluorometric Assay kit is designed for the rapid and sensitive evaluation of SIRT1/Sir2
inhibitors or activators using crude SIRT1/Sir2 fraction or purified SIRT1/Sir2. Additionally, any
cultured primary cell, cell line, or tissue homogenate can be assayed for SIRT1/Sir2 activity with the
CycLex Research Product CycLex SIRT1/Sir2 Deacetylase Fluorometric Assay kit if the appropriate
antibody direct against SIRT1 or Sir2 is used for immunoprecipitation.
Kit Components:
SIRT1 Assay Buffer, Fluoro-Substrate Peptide (0.2 mM), Fluoro-Deacetylated Peptide (0.2 mM), NAD (2 mM), Developer, Recombinant SIRT1, Stop Solution
Shelf Life:
1 year
Target:
SIRT1/Sir2
References
1. Imai S, et al. Nature. 403: 795-800, 2000
2. Landry J et al. Proc Natl Acad Sci U S A 97: 5807-5811, 2000
3. Smith JS, et al. Proc Natl Acad Sci U S A 97: 6658-6663, 2000
4. Vaziri H, et al. Cell. 107: 149-159, 2001
5. Luo J et al. Cell. 107, 137-148, 2001
6. Langley E et al. EMBO J. 21: 2383-2396, 2002
7. Smith J. Trends Cell Biol. 12: 404, 2002
8. Grozinger CM, and Schreiber SL. Chem Biol, 9: 3-16, 2002
9. Sereno, A et al. Antimicrob. Agents Chemother. 49: 808-812, 2005
10. Nicola Ferrara, et al. Rejuvenation Research 11: 139-150, 2008
11. Rameshwar U. et al. Chemical Biology & Drug Design 71: 501-506, 2008
12. Fan Lan, et al. J. Biol. Chem. 283: 27628, 2008
13. Joana TAVARES et al. Biochemical Journal. 415: 377-86, 2008
14. Yue Zhao, et al. Mol. Cell. Biol., Dec 2008; 10.1128/MCB.02123-07.