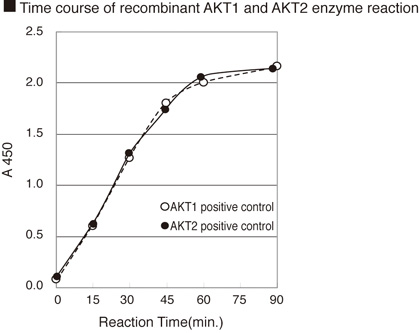
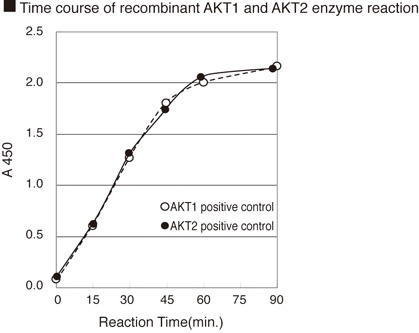
CycLex AKT/PKB Kinase Assay/Inhibitor Screening Kit
| Code | Size | Price |
|---|
| MBL-CY-1168 | 96 Assays | £602.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
4°C
Storage:
4°C. Please refer to datasheet for additional information
Images
Documents
Further Information
Applications:
Other - 1) Screening inhibitors or activators of AKT.
2) Detecting the effects of pharmacological agents on AKT activity.
Background:
The AKT oncogene was isolated from the directly transforming murine retrovirus AKT8, which was
isolated from an AKR mouse thymoma cell line. Staal cloned the human cellular homolog, AKT1 (1).
He found a 20-fold amplification of the AKT1 gene in 1 of 5 gastric adenocarcinomas tested.
Phosphoinositide 3-kinases (PI3Ks) generate specific inositol lipids that have been implicated in the
regulation of cell growth, proliferation, survival, differentiation and cytoskeletal changes. One of the
best characterized targets of PI3K lipid products is the protein kinase AKT or protein kinase B (PKB). In
quiescent cells, AKT resides in the cytosol in a low-activity conformation. Upon cellular stimulation,
AKT is activated through recruitment to cellular membranes by PI3K lipid products and phosphorylation
by 3?-phosphoinositide-dependent kinase-1 (PDK1).
Mammals have three closely related AKT genes, encoding the isoforms AKT1, AKT2 and. AKT3
AKT2 and. AKT3 show 81 and 83% amino acid identity with AKT1 respectively. All AKT isoforms
show a broad tissue distribution and consist of an N-terminal PH domain, a kinase domain and a
C-terminal regulatory tail Two specific sites, one in the kinase domain (Thr308 in AKT1) and the other
in the C-terminal regulatory region (Ser473 in AKT1), need to be phosphorylated for full activation of
these kinases. AKT was among the first proteins known to contain a PH domain, a few years before the
function of this domain came to light. The PH domain of AKT specifically binds PI3K lipid products,
and a firm link between PI3K and AKT signaling has now been established. AKT is cytosolic in
unstimulated cells, and some of it translocates to the plasma membrane upon activation of PI3K, where
it becomes activated (2, 3). Active AKT then appears to detach from the plasma membrane and to
translocate through the cytosol to the nucleus. The mechanism of this translocation is unclear.
AKT1 was found to mediate insulin- and insulin-like growth factor (IGF-1)-induced cellular
responses, such as the inhibition of glycogen synthase kinase-3 (4), the stimulation of glucose uptake (5)
and the promotion of cell survival by inhibiting apoptosis (6). Overexpression of AKT1 or AKT2 is
associated with some human ovarian, pancreatic, and breast carcinomas (7-9).
Description:
The CycLex® AKT/PKB kinase Assay/Inhibitor Screening kit is designed to measure the activities of
purified AKTs for the rapid and sensitive evaluation of inhibitors or activators. The phospho-serine
specific monoclonal antibody used in this assay kit has been demonstrated to recognize the
phospho-serine residue in AKTide-2T, which is efficiently phosphorylated by AKTs.
Gene IDs:
Human: 207 Mouse: 11651
Kit Components:
Microplate, 10x Wash Buffer, Kinase Buffer, 20x ATP, HRP-conjugated Detection Antibody, Substrate Reagent, Stop Solution
Target:
AKT1
References
1. Staal, S. P. Proc. Nat. Acad. Sci. 84, 5034-5037, 1987.
2. Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM,
Hemmings BA. J Biol Chem. 272, 31515-24, 1997
3. Meier, R., Alessi, D. R., Cron, P., Andjelkovic, M. and Hemmings, B. A. J. Biol. Chem. 272,
30491?30497, 1997
4. Cross, D. A.E., Alessi, D. R., Cohen, P., Andjelkovi , M., and Hemmings, B. A. Nature 378, 785-789,
1995
5. Kohn, A. D., Summers, S. A., Birnbaum, M. J., and Roth, R. A. J. Biol. Chem. 271, 31372-31378,
1996
6. Dudek, H., Datta, S. R., Franke, T. F., Birnbaum, M. J., Yao, R., Cooper, G. M., Segal, R. A., Kaplan,
D. R., and Greenberg, M. E. Science 275, 661-665, 1997
7. Cheng, J.Q., Godwin, A. K., Bellacosa, A., Taguchi, T., Franke, T. F., Hamilton, T. C., Tsichlis, P. N.,
and Testa, J. R. Proc. Nat. Acad. Sci. 89, 9267-9271, 1992
8. Bellacosa, A.et al. Int. J. Cancer 64, 280-285, 1995
9. Cheng, J.Q., Ruggeri, B., Klein, W. M., Sonoda, G., Altomare, D. A., Watson, D. K., and Testa, J. R.
Proc. Nat. Acad. Sci. 93, 3636-3641, 1996
10. Toshiyuki Obata, Michael B. Yaffe, German G. Leparc, Elizabeth T. Piro, Hiroshi Maegawa, Atsunori
Kashiwagi, Ryuichi Kikkawa, and Lewis C. J. Biol. Chem. 275: 36108 ? 36115, 2000.
11. Vanhaesebroeck, B.; Alessi, D. R. Biochem. J. 346, 561-576, 2000. (Review)
12. Franke, T. F., Kaplan, D. R., and Cantley, L. C. Cell 88, 435-437, 1997 (Review)
13. Hemmings, B. A. Science 275, 628-630, 1997 (Review)