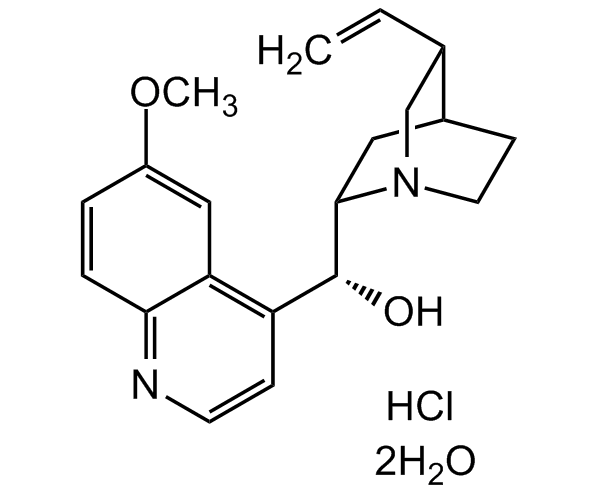
Quinine hydrochloride dihydrate
| Code | Size | Price |
|---|
| CDX-Q0014-G010 | 10 g | £92.00 |
Quantity:
| CDX-Q0014-G050 | 50 g | £335.00 |
Quantity:
| CDX-Q0014-G100 | 100 g | £567.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short term: +20°C, Long term: +20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(8alpha, 9R)-6'-Methoxycinchonan-9-ol monohydrochloride dihydrate
Appearance:
White to off-white solid.
CAS:
6119-47-7
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07, GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H302 - H317 - H334
InChi:
InChI=1S/C20H24N2O2.ClH/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18;/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3;1H/t13-,14-,19-,20+;/m0./s1
InChiKey:
LBSFSRMTJJPTCW-DSXUQNDKSA-N
Long Description:
Chemical. CAS: 6119-47-7. Formula: C20H24N2O2 . HCl . 2H2O. MW: BD9837. Quinine hydrochloride dihydrate is isolated from the cinchona tree's bark, is characterized by a bitter taste and shows antipyretic (fever-reducing), anti-malarial, analgesic (painkilling), anti-viral and anti-inflammatory properties. Quinine also possesses, at high concentrations, antibacterial properties, which suggests it has potential as an antimicrobial adjuvant. It is believed to impede the activity of enzymes associated with nucleic acid and protein biosynthesis and act as an antagonist for specific neurotransmitters, including serotonin. Quinine acts as a competitive inhibitor of monoamine oxidase (MAO), an enzyme that removes neurotransmitters from the brain and a general potasssium channel blocker. Quinine is a flavor component of tonic water and bitter lemon drink mixers. Because of its relatively constant and well-known fluorescence quantum yield, quinine sulfate is used in photochemistry as a popular and common fluorescence standard.
MDL:
MFCD00151248
Molecular Formula:
C20H24N2O2 . HCl . 2H2O
Molecular Weight:
396.91
Package Type:
Vial
Precautions:
P261 - P264 - P270 - P280 - P301 + P312 - P302 + P352
Product Description:
Quinine hydrochloride dihydrate is isolated from the cinchona tree's bark, is characterized by a bitter taste and shows antipyretic (fever-reducing), anti-malarial, analgesic (painkilling), anti-viral and anti-inflammatory properties. Quinine also possesses, at high concentrations, antibacterial properties, which suggests it has potential as an antimicrobial adjuvant. It is believed to impede the activity of enzymes associated with nucleic acid and protein biosynthesis and act as an antagonist for specific neurotransmitters, including serotonin. Quinine acts as a competitive inhibitor of monoamine oxidase (MAO), an enzyme that removes neurotransmitters from the brain and a general potasssium channel blocker. Quinine is a flavor component of tonic water and bitter lemon drink mixers. Because of its relatively constant and well-known fluorescence quantum yield, quinine sulfate is used in photochemistry as a popular and common fluorescence standard.
Purity:
>99% (Titr.)
Signal Word:
Danger
SMILES:
O[C@H](C1=C(C=C(OC)C=C2)C2=NC=C1)[C@H]3[N@](C[C@@H]4C=C)CC[C@H]4C3.Cl
Solubility Chemicals:
Soluble in water (10mg/ml).
Source / Host:
Isolated from plant.
Transportation:
Non-hazardous
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) K.A. Conklin & S.C. Chou; Science 170, 1213 (1970) | (2) D.F. Eaton; Pure Appl. Chem. 60, 1107 (1988) | (3) N. Mitsui, et al.; Chem. Pharm. Bull. 37, 363 (1989) | (4) M. Maki, et al.; Biomed. Res. 30, 137 (2009) | (5) J. Achan, et al.; Malar. J. 10, 144 (2011) | (6) F. Islahudin, et al.; Sci. Rep. 4, 3618 (2014) | (7) S. Malakar, et al.; Virus Res. 255, 171 (2018) | (8) Y. Guo, et al.; Luminescence 34, 450 (2019) | (9) K. Nawara & J. Waluk; Anal. Chem. 91, 5389 (2019) | (10) L.G. Leanse, et al.; J. Infect. Dis. 221, 618 (2020) | (11) U.G. Chandrika & U. Karunarathna; Feat. Assessm. Pain Anaesth. Analg. Chaper 5, 47 (2022)