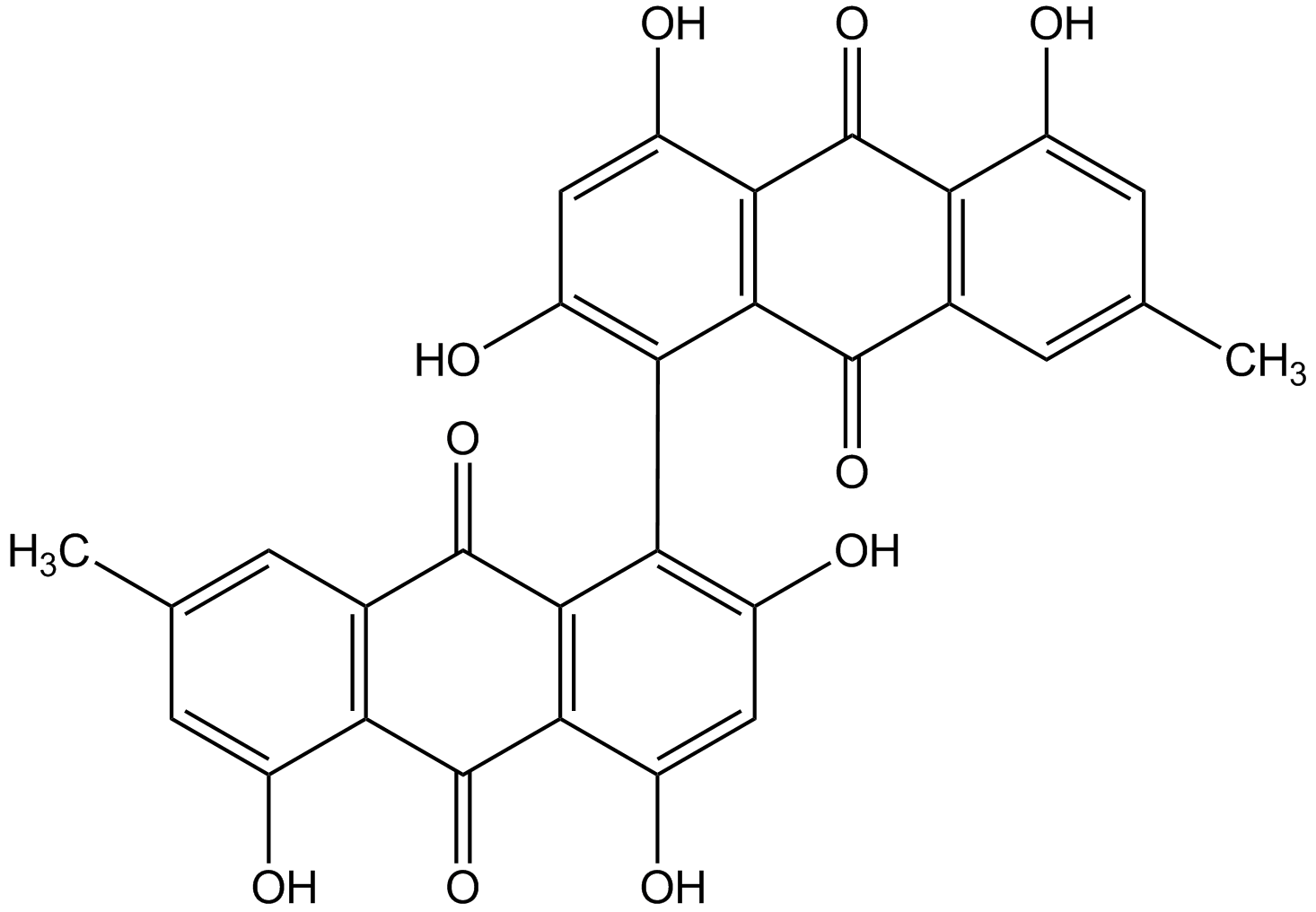
Skyrin
Product Code: AG-CN2-0001
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0001-M001 | 1 mg | £150.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Rhodophyscin; Endothianin; 4,4'-Biemodin
Appearance:
Orange to dark red solid.
CAS:
602-06-2
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light.
InChi:
InChI=1S/C30H18O10/c1-9-3-11-19(13(31)5-9)29(39)23-17(35)7-15(33)21(25(23)27(11)37)22-16(34)8-18(36)24-26(22)28(38)12-4-10(2)6-14(32)20(12)30(24)40/h3-8,31-36H,1-2H3
InChiKey:
MQSXZQXHIJMNAF-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 602-06-2. Formula: C30H18O10. MW: 538.5. Non-peptidic anti-diabetic agent. Receptor-selective glucagon antagonist. Free radical species (?OH, R?) and singlet oxygen (1O2) scavenger. Mycotoxin. Cytotoxic. Antioxidant. Inhibitor of botulinum neurotoxin serotype A (BoNTA). Anti-MRSA compound. Antibiotic.
MDL:
MFCD00597057
Molecular Formula:
C30H18O10
Molecular Weight:
538.5
Package Type:
Vial
Product Description:
Non-peptidic anti-diabetic agent [1]. Receptor-selective glucagon antagonist [1]. Free radical species (?OH, R?) and singlet oxygen (1O2) scavenger [2]. Mycotoxin [3, 4]. Cytotoxic [3, 4]. Antioxidant [2]. Inhibitor of botulinum neurotoxin serotype A (BoNTA) [5]. Anti-MRSA compound [6]. Antibiotic [7].
Purity:
>97% (HPLC)
SMILES:
CC1=CC2=C(C(O)=C1)C(=O)C1=C(C2=O)C(=C(O)C=C1O)C1=C(O)C=C(O)C2=C1C(=O)C1=C(C(O)=CC(C)=C1)C2=O
Solubility Chemicals:
Soluble in 100% ethanol or DMSO.
Source / Host:
Isolated from fungus Talaromyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Effects of skyrin, a receptor-selective glucagon antagonist, in rat and human hepatocytes: J.C. Parker, et al.; Diabetes 49, 2079 (2000) | Antioxidant and scavenging activity of skyrin on free radical and some reactive oxygen species: F. Vargas, et al.; Avances en Quimica 3, 7 (2008) | The bioactive metabolites of the mangrove endophytic fungus Talaromyces sp. ZH-154 isolated from Kandelia candel (L.) Druce: F. Liu, et al.; Planta Med. 76, 185 (2010) | A comparative study on cytotoxicities and biochemical properties of anthraquinone mycotoxins emodin and skyrin from Penicillium islandicum Sopp: K. Kawai, et al.; Toxicol. Lett. 20, 155 (1984) | Fungal bis-naphthopyrones as inhibitors of Botulinum neurotoxin serotype A: J. H. Cardellina II, e. Al.; ACS Med. Chem. Lett. 3, 387 (2012) | Atropisomeric dihydroanthracenones as inhibitors of multiresistant Straphylococcus aureus: R. Bara, et al.; J. Med. Chem. 56, 3257 (2013) | Antibiotically active metabolites from Talaromyces wortmannii, an endophyte of Aloe vera: R. Bara, et al.; J. Antibiot. 66, 491 (2013)