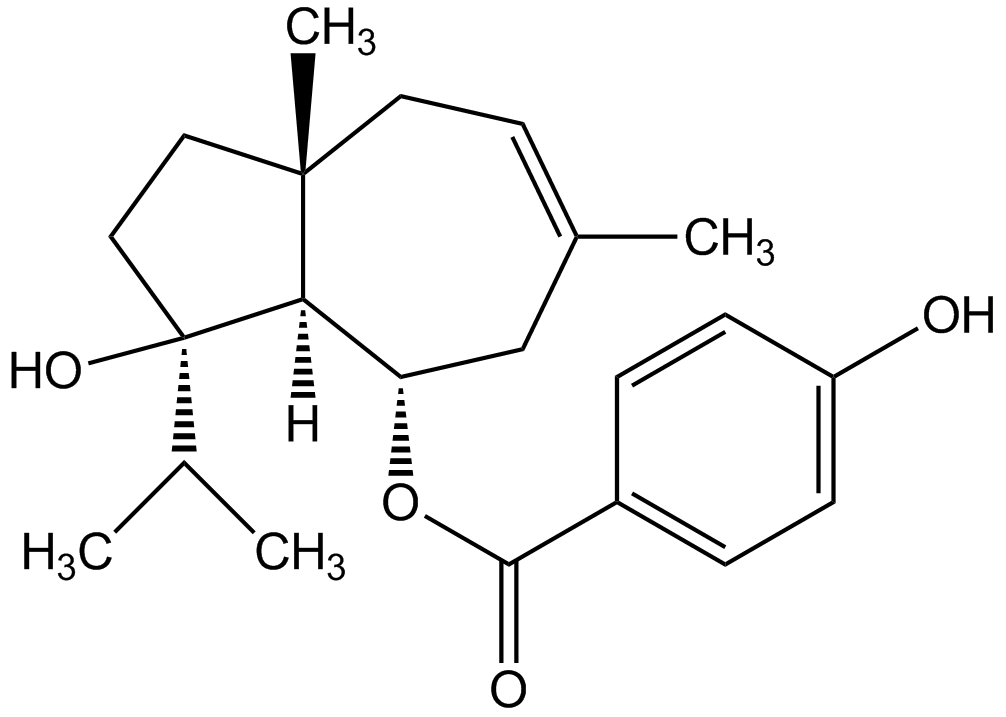
Ferutinin (high purity)
Product Code: AG-CN2-0007
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0007-M001 | 1 mg | £50.00 |
Quantity:
| AG-CN2-0007-M005 | 5 mg | £130.00 |
Quantity:
| AG-CN2-0007-M010 | 10 mg | £200.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
4-Oxy-6-(4-oxybenzoyloxy)dauc-8,9-en; Tefestrol
Appearance:
White to off-white solid.
CAS:
41743-44-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep under inert gas.Protect from light.Protect from moisture and oxygen.
InChi:
InChI=1S/C22H30O4/c1-14(2)22(25)12-11-21(4)10-9-15(3)13-18(19(21)22)26-20(24)16-5-7-17(23)8-6-16/h5-9,14,18-19,23,25H,10-13H2,1-4H3/t18-,19+,21-,22+/m0/s1
InChiKey:
CYSHNJQMYORNJI-YUVXSKOASA-N
Long Description:
Chemical. CAS: 41743-44-6. Formula: C22H30O4. MW: 358.5. Semisynthetic. Potent, naturally occuring non-steroid estrogenic compound. Tool to promote mitochondrial calcium overload and to promote calcium-dependent opening of the mitochondrial permeability transition pore (mPTP). Strong agonist for estrogen receptor (ER)alpha and agonist/antagonist for ERbeta. Calcium ionophoretic Apoptosis modulator. Antiproliferative. Increases nitric oxide synthase activity and phosphoinositides breakdown in nervous tissue. Shows aphrodisiac and anti-sexual impotence activities. Anti-osteoporotic. Prevents osteoporosis caused by severe estrogen deficiency. Modest AChE inhibitor.
MDL:
MFCD18072196
Molecular Formula:
C22H30O4
Molecular Weight:
358.5
Package Type:
Vial
Product Description:
Potent, naturally occuring non-steroid estrogenic compound. Tool to promote mitochondrial calcium overload and to promote calcium-dependent opening of the mitochondrial permeability transition pore (mPTP) [1, 3, 4]. Strong agonist for estrogen receptor (ER)alpha and agonist/antagonist for ERbeta [2, 8]. Calcium ionophoretic [4, 11] Apoptosis modulator [4]. Antiproliferative [5, 7]. Increases nitric oxide synthase activity and phosphoinositides breakdown in nervous tissue [6]. Shows aphrodisiac and anti-sexual impotence activities [6]. Anti-osteoporotic. Prevents osteoporosis caused by severe estrogen deficiency [9]. Modest AChE inhibitor [10].
Purity:
>98% (HPLC)
SMILES:
[H][C@@]12[C@H](CC(C)=CC[C@@]1(C)CC[C@@]2(O)C(C)C)OC(=O)C1=CC=C(O)C=C1
Solubility Chemicals:
Soluble in DMSO, acetone, dichloromethane or ethyl acetate.
Source / Host:
Semisynthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
Ionophoretic properties of ferutinin: M.V. Zamaraeva, et al.; Cell Calcium 22, 235 (1997) | Terpenoids found in the umbelliferae family act as agonists/antagonists for ER(alpha) and ERbeta: differential transcription activity between ferutinine-liganded ER(alpha) and ERbeta: K. Ikeda, et al.; BBRC 291, 354 (2002) | Actions of ionomycin, 4-BrA23187 and a novel electrogenic Ca2+ ionophore on mitochondria in intact cells: A.Y. Abramov & M.R. Duchen; Cell Calcium 33, 101 (2003) | Calcium ionophoretic and apoptotic effects of ferutinin in the human Jurkat T-cell line: A. Macho, et al.; Biochem. Pharmacol. 68, 875 (2004) | Antiproliferative effects of daucane esters from Ferula communis and F. arrigonii on human colon cancer cell lines: F. Poli, et al.; Phytother. Res. 19, 152 (2005) | Ferutinin stimulates nitric oxide synthase activity in median eminence of the rat: T. Colman-Saizarbitoria, et al.; J. Ethnopharmacol. 106, 327 (2006) | Activity of elaeochytrin A from Ferula elaeochytris on leukemia cell lines: R. Alkhatib, et al.; Phytochemistry 69, 2979 (2008) | The phytoestrogen ferutinin affects female sexual behavior modulating ERalpha expression in the hypothalamus: P. Zanoli, et al.; Behav. Brain Res. 199, 283 (2009) | Influence of ferutinin on bone metabolism in ovariectomized rats. II: role in recovering osteoporosis: M. Ferretti, et al.; J. Anat. 217, 4 (2010) | Identification of non-alkaloid acetylcholinesterase inhibitors from Ferulago campestris (Besser) Grecescu (Apiaceae): S. Dall'acqua, et al.; Fitoterapia 81, 1208 (2010) | Ferutinine structure: A.I. Saidkhodjaev, et al.; Chem. Nat. Comp. 1, 28 (1973)
Related Products
| Product Name | Product Code | Supplier | Beauvericin | AG-CN2-0043 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rubratoxin A | AG-CN2-0092 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||