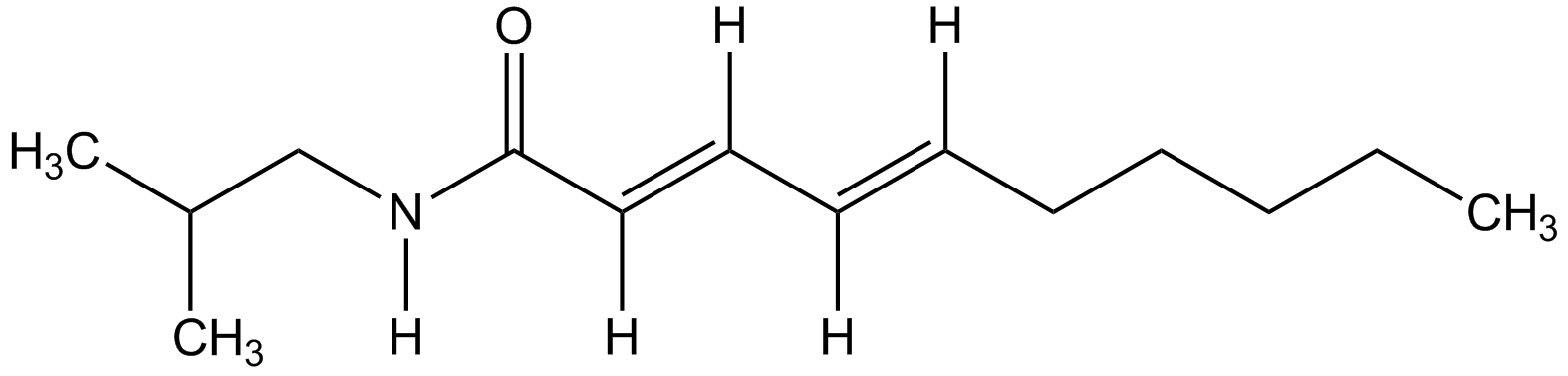
Pellitorine
Product Code: AG-CN2-0009
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0009-M001 | 1 mg | £40.00 |
Quantity:
| AG-CN2-0009-M005 | 5 mg | £85.00 |
Quantity:
| AG-CN2-0009-M025 | 25 mg | £290.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
BLUE ICE
Storage:
Short Term Storage: +4?C. Long Term Storage: -20?C
Images
Documents
Further Information
Alternate Names/Synonyms:
(2E,4E)-N-(2-Methylpropyl)-2,4-decadienamide; BRN 1725967
Appearance:
Yellow to beige solid.
CAS:
18836-52-7
EClass:
32160000
Form (Short):
solid
InChi:
InChI=1S/C14H25NO/c1-4-5-6-7-8-9-10-11-14(16)15-12-13(2)3/h8-11,13H,4-7,12H2,1-3H3,(H,15,16)/b9-8+,11-10+
InChiKey:
MAGQQZHFHJDIRE-BNFZFUHLSA-N
Long Description:
Chemical. CAS: 18836-52-7. Formula: C14H25NO. MW: 223.4. Synthetic. Originally isolated from roots of Anacyclus pryrethrum and fruits of Piper nigrum. Tingling-inducing agent. Excellent stable model compound for sensory studies. Exerts same profile as the unstable compound hydroxy-alpha-sanshool. Shows larvicidal, antimycobacterial and antituberculosis activity. alpha-Glucosidase inhibitor used in diabetes mellitus, cancer, infection and inflammatory research. ACAT (Acyl-CoA cholesteryl acyl transferase) inhibitor. Potential anti-cancer lead compound. Anti-thrombotic. Anti-septic. Antiprotozoal, antimalarial activity.
MDL:
MFCD01735995
Molecular Formula:
C14H25NO
Molecular Weight:
223.4
Package Type:
Vial
Product Description:
Tingling-inducing agent. Excellent stable model compound for sensory studies [3]. Exerts same profile as the unstable compound hydroxy-alpha-sanshool. Shows larvicidal, antimycobacterial and antituberculosis activity [1, 2, 4]. alpha-Glucosidase inhibitor used in diabetes mellitus, cancer, infection and inflammatory research [5]. ACAT (Acyl-CoA cholesteryl acyl transferase) inhibitor [6]. Potential anti-cancer lead compound [7]. Anti-thrombotic [8]. Anti-septic [9]. Antiprotozoal, antimalarial activity [10]. Antagonist of the TRPV1 [11].
Purity:
>97% (HPLC)
SMILES:
[H]N(CC(C)C)C(=O)C([H])=C(/[H])C([H])=C(/[H])CCCCC
Solubility Chemicals:
Soluble in 100% ethanol, methanol, DMSO or dichloromethane. Sparingly soluble in water.
Source / Host:
Synthetic. Originally isolated from roots of Anacyclus pryrethrum and fruits of Piper nigrum.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20?C.
References
Larvicidal activity of isobutylamides identified in Piper nigrum fruits against three mosquito species: I.K. Park, et al.; J. Agric. Food Chem. 50, 1866 (2002) | Chemical constituents and bioactivity of Piper sarmentosum: T. Rukachaisirikul, et al.; J. Ethnopharmacol. 93, 173 (2004) | Stereoselective Enzymatic Synthesis of cis-Pellitorine, a Taste Active Alkamide Naturally Occurring in Tarragon: P. J. Ley, et al.; Eur. J. Org. Chem. 2004, 5135 | Chemical constituents of the roots of Piper sarmentosum: P. Tuntiwachwuttikul, et al.; Chem. Pharm. Bull. (Tokyo) 54, 149 (2006) | HPLC assisted chemobiological standardization of alpha-glucosidase-I enzyme inhibitory constituents from Piper longum Linn - An Indian medicinal plant: S.V. Pullela, et al.; J. Ethnopharmacol. 108, 445 (2006) | ACAT inhibition of alkamides identified in the fruits of Piper nigrum: M.C. Rho, et al.; Phytochemistry 68, 899 (2007) | Pellitorine, a potential anti-cancer lead compound against HL6 and MCT-7 cell lines and microbial transformation of piperine from Piper Nigrum: G.C. Ee, et al.; Molecules 15, 2398 (2010) | Antithrombotic activities of pellitorine in vitro and in vivo: S.K. Ku, et al.; Fitoterapia 91, 1 (2013) | Anti-septic effects of pellitorine in HMGB1-induced inflammatory responses in vitro and in vivo: S.K. Ku, et al.; Inflammation 37, 338 (2014) | Antiprotozoal activity of Achillea ptarmica (Asteraceae) and its main alkamide constituents: J.B. Althaus, et al.; Molecules 19, 6428 (2014) | Pellitorine, an extract of Tetradium daniellii, is an antagonist of the ion channel TRPV1: Z. Olah, et al.; Phytomed. 34, 44 (2017)