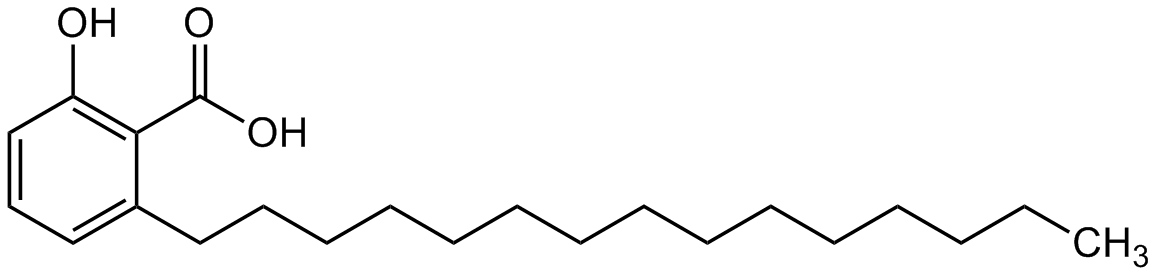
Anacardic acid
| Code | Size | Price |
|---|
| AG-CR1-0046-M005 | 5 mg | £45.00 |
Quantity:
| AG-CR1-0046-M025 | 25 mg | £130.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
+4°C
Storage:
4°C
Images
Further Information
Alternate Names/Synonyms:
2-Hydroxy-6-pentadecylbenzoic acid; 6-Pentadecylsalicylic acid; AA
Appearance:
White to off-white solid.
CAS:
16611-84-0
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
After reconstitution, prepare aliquots and store at -20°C.Protect from light.
Hazards:
H317, H319
InChi:
InChI=1S/C22H36O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h15,17-18,23H,2-14,16H2,1H3,(H,24,25)
InChiKey:
ADFWQBGTDJIESE-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 16611-84-0. Formula: C22H36O3. MW: 348.5. Potent histone acetyltransferase (HAT) inhibitor. Antibacterial. SUMOylation inhibitor. Lipoxygenase inhibitor. NF-kappaB inhibitor. Anti-tumor compound. Anti-inflammatory. Apoptosis inducer. Antiproliferative.
MDL:
MFCD07368254
Molecular Formula:
C22H36O3
Molecular Weight:
348.5
Other data:
Stock solutions are stable for up to 3 months when stored at -20°C.
Package Type:
Vial
Precautions:
P261, P272, P280, P302, P352, P333, P313
Product Description:
Potent histone acetyltransferase (HAT) inhibitor [1, 2]. Antibacterial [3]. SUMOylation inhibitor [4]. Lipoxygenase inhibitor [5]. NF-kappaB inhibitor [6]. Anti-tumor compound [2, 6-8]. Anti-inflammatory [6]. Apoptosis inducer [6, 7]. Antiproliferative [7].
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
CCCCCCCCCCCCCCCC1=CC=CC(O)=C1C(O)=O
Solubility Chemicals:
Soluble in 100% ethanol, methanol, DMSO, dichloromethane or ethylacetate.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
Small molecule modulators of histone acetyltransferase p300: K. Balasubramanyam, et al.; J. Biol. Chem. 278, 19134 (2003) | Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation: Y. Sun, et al.; FEBS Lett. 580, 4353 (2006) | Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin-resistant Staphylococcus aureus: H. Muroi & I. Kubo; J. Appl. Bacteriol. 80, 387 (1996) | Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate: I. Fukuda, et al.; Chem. Biol. 16, 133 (2009) | Inhibition of lipoxygenase and prostaglandin endoperoxide synthase by anacardic acids: R. Grazzini, et al.; BBRC 176, 775 (1991) | Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis: B. Sung, et al.; Blood 111, 4880 (2008) | Anacardic acid induces caspase-independent apoptosis and radiosensitizes pituitary adenoma cells: S. Sukumari-Ramesh, et al.; J. Neurosurg. 114, 1681 (2011) | Anacardic acid inhibits estrogen receptor alpha-DNA binding and reduces target gene transcription and breast cancer cell proliferation: D.J. Schultz, et al.; Mol. Cancer Ther. 9, 594 (2010)