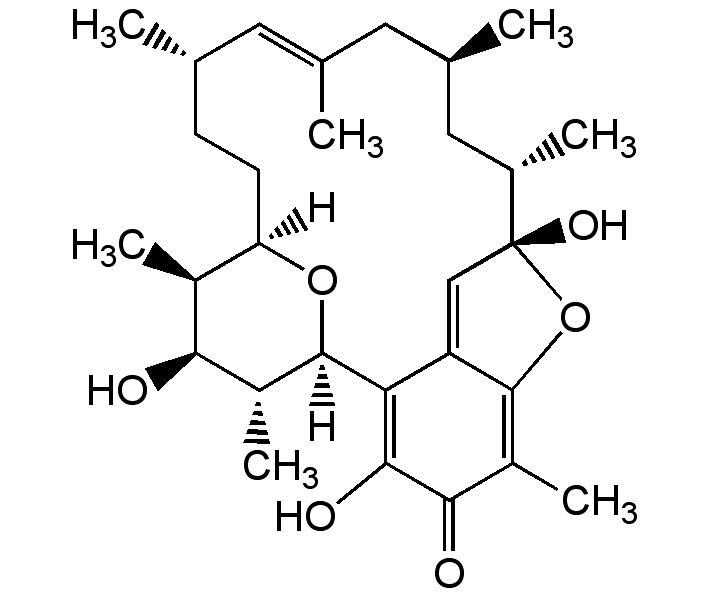
Kendomycin
| Code | Size | Price |
|---|
| BVT-0001-C100 | 100 ug | £85.00 |
Quantity:
| BVT-0001-C500 | 500 ug | £160.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Further Information
Alternate Names/Synonyms:
(-)-TAN 2162
Appearance:
Yellow powder.
CAS:
183202-73-5
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light.
Hazards:
H302, H312, H319
InChi:
InChI=1S/C29H42O6/c1-14-8-9-22-18(5)24(30)19(6)28(34-22)23-21-13-29(33,17(4)12-16(3)11-15(2)10-14)35-27(21)20(7)25(31)26(23)32/h10,13-14,16-19,22,24,28,30,32-33H,8-9,11-12H2,1-7H3/b15-10+/t14-,16+,17-,18-,19+,22+,24-,28+,29+/m0/s1
InChiKey:
HKLDUJXJTQJSEJ-OLXNOMCWSA-N
Long Description:
Chemical. CAS: 183202-73-5. Formula: C29H42O6. MW: 486.6. Isolated from Streptomyces violaceoruber. Antibiotic. Potent endothelin receptor antagonist. Anti-osteoporotic. Antibacterial. Cytotoxic. Mediates its cytotoxic effects, at least in part, through proteasome inhibition.
MDL:
MFCD14635192
Molecular Formula:
C29H42O6
Molecular Weight:
486.6
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Antibiotic. Potent endothelin receptor antagonist. Anti-osteoporotic. Antibacterial. Anticancer agent. Cytotoxic against several carcinoma cell lines (GI50<100nM). 20S proteasome inhibitor. Induces apoptosis by inhibition of chymotrypsin-like proteasome activity.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
[H][C@]12CC[C@H](C)C=C(C)C[C@@H](C)C[C@H](C)[C@]3(O)OC4=C(C)C(=O)C(O)=C(C4=C3)[C@]([H])(O1)[C@H](C)[C@@H](O)[C@H]2C
Solubility Chemicals:
Slightly soluble in methanol or DMSO.
Source / Host:
Isolated from Streptomyces violaceoruber.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Structure and biosynthesis of kendomycin, a carboxylic ansa-compound from Streptomyces: H.B. Bode & A. Zeeck; J. Chem. Soc. Perkin Trans 1, 323 and 2665 (2000) | Evidence for the mode of action of the highly cytotoxic Streptomyces polyketide kendomycin: Y.A. Elnakady, et al.; ChemBioChem. 8, 1261 (2007) | Formal Synthesis of (?)-Kendomycin Featuring a Prins-Cyclization To Construct the Macrocycle: K.B. Bahnck & S.D. Rychnovsky; JACS 130, 13177 (2008) | Interactions of the natural product kendomycin and the 20S proteasome: P. Beck, et al.; J. Mol. Biol. 426, 3108 (2014)
Related Products
| Product Name | Product Code | Supplier | Echinosporin | BVT-0006 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|