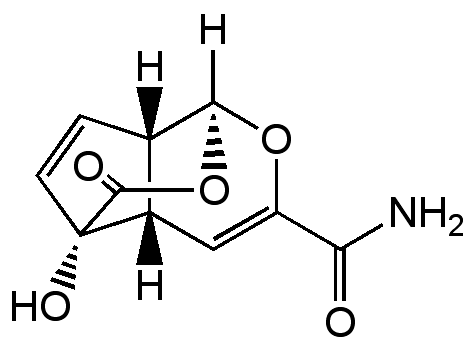
Echinosporin
| Code | Size | Price |
|---|
| BVT-0006-M001 | 1 mg | £150.00 |
Quantity:
| BVT-0006-M005 | 5 mg | £500.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NSC357683; XK 213; (1S,4aS,5S,7aS)-5-Hydroxy-8-oxo-1,4a,5,7a-tetrahydro-1,5-(epoxymethano)cyclopenta[c] pyran-3-carboxamide
Appearance:
White to off-white solid.
CAS:
79127-35-8
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302, H312, H319, H332
InChi:
InChI=1S/C10H9NO5/c11-7(12)6-3-5-4-1-2-10(5,14)9(13)16-8(4)15-6/h1-5,8,14H,(H2,11,12)/t4-,5-,8-,10-/m0/s1
InChiKey:
OXSZHYWOGQJUST-PDXIVQBHSA-N
Long Description:
Chemical. CAS: 79127-35-8. Formula: C10H9NO5. MW: 223.2. Isolated from Streptomyces sp. Antibiotic. Cell cycle inhibitor at the G(2)/M phase. Antitumor compound. Apoptosis inducer.
MDL:
MFCD01939575
Molecular Formula:
C10H9NO5
Molecular Weight:
223.2
Package Type:
Plastic Vial
Precautions:
P261, P270, P280, P301, P312, P302, P352, P312
Product Description:
Antibiotic. Cell cycle inhibitor at the G(2)/M phase. Antitumor compound. Apoptosis inducer.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
[H][C@@]12C=C[C@@]3(O)C(=O)O[C@]1([H])OC(=C[C@@]23[H])C(N)=O
Solubility Chemicals:
Soluble in water, methanol or dimethylformamide.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. Stock solutions are stable for at least 3 months when stored at -20°C. After reconstitution protect from light at -20°C.
References
A new antibiotic echinosporin (XK-213) - producing organism, isolation and characterization: T. Sato, et al.; J. Antibiot. 35, 266 (1982) | Antitumor activity of echinosporin: M. Morimoto and R. Imai; J. Antibiot. 38, 490 (1985) | Approaches to the total synthesis of the antitumor antibiotic echinosporin: M. A. Kinsella; J. Org. Chem. 55, 105 (1990) | Applications of an asymmetric [2 + 2]-photocycloaddition. Total synthesis of (-)-echinosporin. Construction of an advanced 11-deoxyprostaglandin intermediate: A. B. Smith; J. Am. Chem. Soc. 114, 2567 (1992) | Biosynthesis of the antibiotic echinosporin by a novel branch of the shikimate pathway: A. Dubeler; Eur. J. Org. Chem. 2002, 983 (2002) | Echinosporins as new cell cycle inhibitors and apoptosis inducers from marine-derived Streptomyces albogriseolus: C.B. Cui, et al.; Fitoterapia 78, 238 (2007) | A new stereocontrolled synthetic route to (-)-echinosporin from D-glucose via padwa allenylsulfone [3 + 2]-anionic cycloadditive elimination: J.T. Flasz, et al.; Org. Lett. 14, 3024 (2012) | Interaction of marine streptomyces compounds with selected cancer drug target proteins by in silico molecular docking studies: A.R. Lankapalli & K. Kannabiran; Interdiscipl. Sci. Comput. Life Sci. 5, 37 (2013)
Related Products
| Product Name | Product Code | Supplier | Kendomycin | BVT-0001 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|