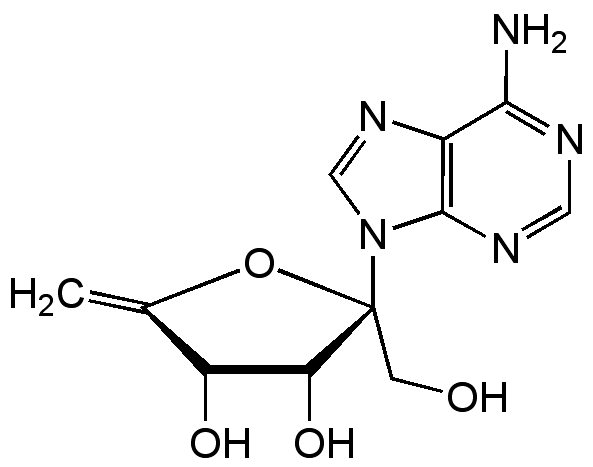
Decoyinine
| Code | Size | Price |
|---|
| BVT-0030-M001 | 1 mg | £85.00 |
Quantity:
| BVT-0030-M005 | 5 mg | £310.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Angustmycin A; U-7984; 9-(6-Deoxy-D-beta-erythro-hex-5-en-2-ulofuranosyl)-adenine
Appearance:
White to off-white solid.
CAS:
08/04/2004
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light when in solution.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C11H13N5O4/c1-5-7(18)8(19)11(2-17,20-5)16-4-15-6-9(12)13-3-14-10(6)16/h3-4,7-8,17-19H,1-2H2,(H2,12,13,14)/t7-,8-,11-/m1/s1
InChiKey:
UZSSGAOAYPICBZ-SOCHQFKDSA-N
Long Description:
Chemical. CAS: 2004-04-8. Formula: C11H13N5O4. MW: 279.3. Isolated from Streptomyces sp. S 2113. Nucleoside antibiotic. Antitumor compound. Xanthosine monophosphate (XMP) aminase inhibitor. RNA synthesis inhibitor. Specific GMP synthase inhibitor. Reduces intracellular GTP levels.
MDL:
MFCD31689304
Molecular Formula:
C11H13N5O4
Molecular Weight:
279.3
Package Type:
Plastic Vial
Precautions:
P261, P271, P280, P312
Product Description:
Nucleoside antibiotic. Antitumor compound. Xanthosine monophosphate (XMP) aminase inhibitor. RNA synthesis inhibitor. Specific GMP synthase inhibitor. Reduces intracellular GTP levels.
Purity:
>98% (NMR)
Signal word:
Warning
SMILES:
NC1=NC=NC2=C1N=CN2[C@]1(CO)OC(=C)C(O)[C@@H]1O
Solubility Chemicals:
Soluble in DMSO (warm), water or 100% ethanol: poorly soluble in methanol.
Source / Host:
Isolated from Streptomyces sp. S 2113.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Studies on a new antibiotic, angustmycin: H. Sakai, et al.; J. Antibiot. 7, 116 (1954) | Studies on a new antibiotic, angustmycin. I: H. Yuntsen, et al.; J. Antibiot. 7, 113 (1954) | Studies on angustmycin. III: H. Yuntsen, et al.; J. Antibiot. 9, 195 (1956) | On the studies of angustmycins. VI. Chemical structure of angustmycin A: H. Yuntsen; J. Antibiot. 11, 79 (1958) | Purine nucleosides. XXII. The synthesis of angustmycin A (decoyinine) and related unsaturated nucleosides: J.R. McCarthy, Jr. et al.; J. Am. Chem. Soc. 90, 4993 (1968) | Effects of several tumor-inhibitory antibiotics on immunological responses: H. Yamaki, et al.; J. Antibiot. 22, 315 (1969) | Halo sugar nucleosides. 5. Synthesis of angustmycin A and some base analogues: E.J. Prisbe, et al.; J. Org. Chem. 41, 1836 (1976) | A decrease in GTP content is associated with aerial mycelium formation in Streptomyces MA406-A-1: K. Ochi; J. Gen. Microbiol. 132, 299 (1986) | Extracellular control of spore formation in Bacillus subtilis: A.D. Grossman & R. Losick; PNAS 85, 4369 (1988) | Nucleoside antibiotics: structure, biological activity, and biosynthesis: K. Isono; J. Antibiot. 41, 1711 (1988) | A target for carbon source-dependent negative regulation of the citB promoter of Bacillus subtilis: A. Fouret & A.L. Sonenshein; J. Bacteriol. 172, 835 (1990) | Sporulation of Streptomyces venezuelae in submerged cultures: M.A. Glazebrook, et al.; J. Gen. Microbiol. 136, 581 (1990) | Conformational studies on some Cl'-branched beta-D-nucleosides by 1H-NMR spectroscopy and molecular mechanics calculations: J. Plavec, et al.; J. Biochem. Biophys. Meth. 26, 317 (1993) | Biochemical characterization of human GMP synthetase: J. Nakamura & L. Lou; J. Biol. Chem. 270, 7347 (1995) | Guanine nucleotides guanosine 5'-diphosphate 3'-diphosphate and GTP co-operatively regulate the production of an antibiotic bacilysin in Bacillus subtilis: T. Inaoka, et al.; J. Biol. Chem. 278, 2169 (2003) | Expression of kinA and kinB of Bacillus subtilis, necessary for sporulation initiation, is under positive stringent transcription control: S. Tojo, et al.; J. Bacteriol. 195, 1656 (2013) | Pharmacological targeting of guanosine monophosphate synthase suppresses melanoma cell invasion and tumorigenicity: A. Bianchi-Smiraglia, et al.; Cell Death Differ. 22, 1858 (2015)
Related Products
| Product Name | Product Code | Supplier | Mensacarcin | BVT-0028 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspyrone | BVT-0029 | Bioviotica | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CJ-21058 | BVT-0064 | Bioviotica | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||