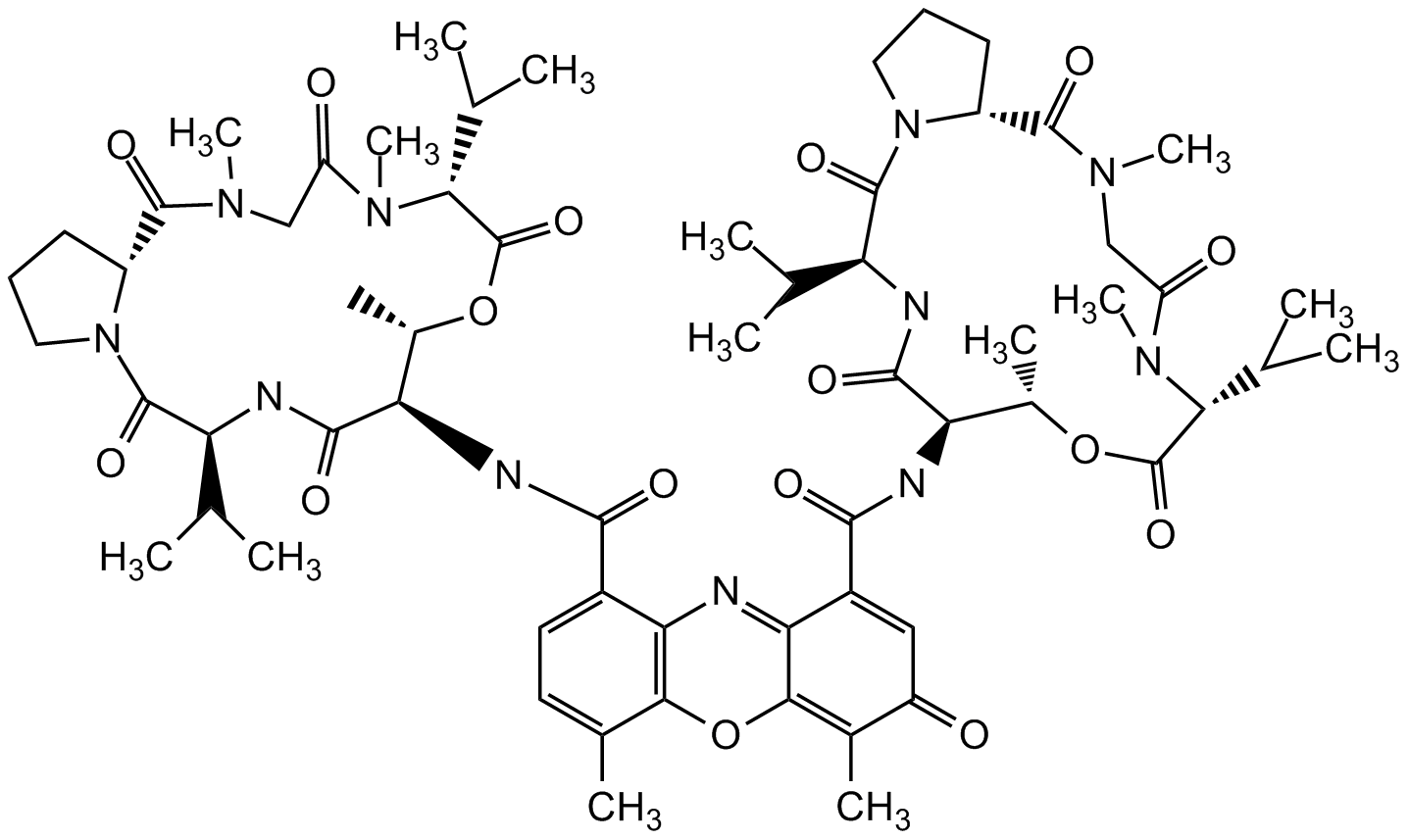
Actinomycin D
| Code | Size | Price |
|---|
| BVT-0089-M005 | 5 mg | £65.00 |
Quantity:
| BVT-0089-M025 | 25 mg | £170.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Actinomycin IV; Actinomycin C1; Dactinomycin
Appearance:
Red shiny crystals.
CAS:
50-76-0
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Keep cool and dry.Protect from light when in solution.
Hazards:
H300, H312, H332
InChi:
InChI=1S/C62H85N11O16/c1-28(2)43-59(83)72-23-17-19-38(72)57(81)68(13)26-41(75)70(15)49(30(5)6)61(85)87-34(11)45(55(79)64-43)66-53(77)36-22-21-32(9)51-47(36)63-48-37(25-40(74)33(10)52(48)89-51)54(78)67-46-35(12)88-62(86)50(31(7)8)71(16)42(76)27-69(14)58(82)39-20-18-24-73(39)60(84)44(29(3)4)65-56(46)80/h21-22,25,28-31,34-35,38-39,43-46,49-50H,17-20,23-24,26-27H2,1-16H3,(H,64,79)(H,65,80)(H,66,77)(H,67,78)/t34-,35-,38+,39+,43-,44-,45+,46+,49+,50+/m0/s1
InChiKey:
ZOWMRHVAQGTFTG-IZRKADTCSA-N
Long Description:
Chemical. CAS: 50-76-0. Formula: C62H86N12O16. MW: 1255.4. Isolated from Streptomyces parvulus. Antibiotic. Antitumor compound. Cytotoxic. Apoptosis inducer. RNA synthesis inhibitor. DNA intercalating agent. Mcl-1 expression inhibitor p53 pathway activator.
MDL:
MFCD00005033
Molecular Formula:
C62H86N12O16
Molecular Weight:
1255.4
Other data:
Sensitive to strong acids and bases.
Package Type:
Plastic Vial
PG:
III
Precautions:
P262, P280, P301, P310, P302, P350, P310, P405
Product Description:
Antibiotic. Antitumor compound [2]. Cytotoxic [2]. Apoptosis inducer [1, 2, 6, 7]. RNA synthesis inhibitor [4]. DNA intercalating agent [3, 5]. Mcl-1 expression inhibitor [8] p53 pathway activator [9].
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
CC(C)[C@@H]1NC(=O)[C@H](NC(=O)C2=CC=C(C)C3=C2N=C2C(O3)=C(C)C(=O)C=C2C(=O)N[C@@H]2[C@H](C)OC(=O)[C@@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@H]3CCCN3C(=O)[C@@H](NC2=O)C(C)C)[C@H](C)OC(=O)[C@@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@H]2CCCN2C1=O
Solubility Chemicals:
Soluble in DMSO, 100% ethanol or methanol.
Source / Host:
Isolated from Streptomyces parvulus.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C. After reconstitution protect from light at -20°C.
References
Apoptosis induced by Actinomycin D, Camptothecin or Aphidicolin can occur in all phases of the cell cycle: J.M. Glynn, et al.; Biochem. Soc. Trans. 20, 84S (1992) | Actinomycin D induces apoptosis and inhibits growth of pancreatic cancer cells: J. Kleeff, et al.; Int. J. Cancer 86, 399 (2000) | Influence of DNA base sequence on the binding energetics of actinomycin D: S.A. Bailey, et al.; Biochemistry 32, 5881 (1993) | Effect of actinomycin on RNA synthesis and antibody formation in the anamnestic response in vitro: J.D. Smiley, et al.; J. Exp. Med. 119, 881 (1964) | NMR Solution Structure of a DNA-Actinomycin D Complex Containing a Non-Hydrogen-Bonding Pair in the Binding Site: S.L. Cravens, et al.; J. Am. Chem. Soc. 132, 17588 (2010) | Actinomycin D upregulates proapoptotic protein Puma and downregulates Bcl-2 mRNA in normal peripheral blood lymphocytes: I. Kalousek, et al.; Anticancer Drugs 18, 763 (2007) | Actinomycin D enhances TRAIL-induced caspase-dependent and -independent apoptosis in SH-SY5Y neuroblastoma cells: M.J. Wang, et al.; Neurosci. Res. 59, 40 (2007) | Actinomycin D synergistically enhances the efficacy of the BH3 mimetic ABT-737 by downregulating Mcl-1 expression: K.E. Olberding, et al.; Cancer Biol. Ther. 10, 930 (2010) | Specific activation of the p53 pathway by low dose actinomycin D: M. L. Choong, et al.; Cell Cycle 8, 2810 (2009) | Restoring NAD+ by NAMPT is essential for the SIRT1/p53-mediated survival of UVA- and UVB-irradiated epidermal keratinocytes: T. Katayoshi, et al.; J. Photochem. Photobiol. B 221, 112238 (2021)