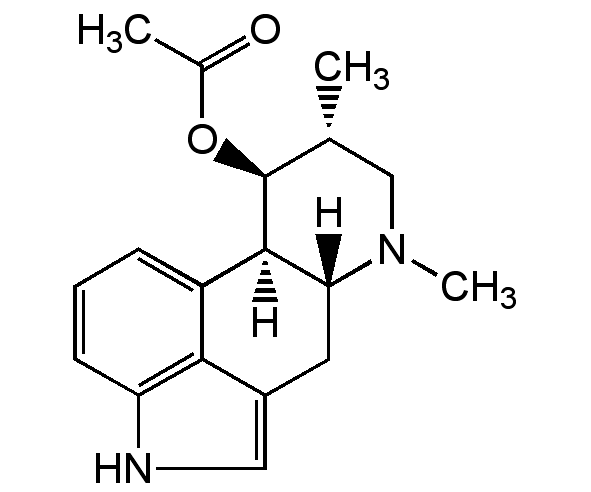
Fumigaclavine A
| Code | Size | Price |
|---|
| BVT-0090-M001 | 1 mg | £160.00 |
Quantity:
| BVT-0090-M005 | 5 mg | £460.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(6aR,9R,10S,10aR)-7,9-Dimethyl-4,6,6a,7,8,9,10,-10a-octahydroindolo[4,3-fg]quinolin-10-yl acetate
Appearance:
Beige to brown solid.
CAS:
6879-59-0
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302, H312, H319
InChi:
InChI=1S/C18H22N2O2/c1-10-9-20(3)15-7-12-8-19-14-6-4-5-13(16(12)14)17(15)18(10)22-11(2)21/h4-6,8,10,15,17-19H,7,9H2,1-3H3/t10-,15-,17-,18+/m1/s1
InChiKey:
GJSSYQDXZLZOLR-ONUGHKICSA-N
Long Description:
Chemical. CAS: 6879-59-0. Formula: C18H22N2O2. MW: 298.4. Isolated from Aspergillus sp. Ergot alkaloid. Mycotoxin.
MDL:
MFCD01729203
Molecular Formula:
C18H22N2O2
Molecular Weight:
298.4
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Ergot alkaloid. Mycotoxin. Fumigaclavine A is convertet into fumigaclavine C by the enzyme Reverse Prenyltransferase FgaPT1.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
[H][C@@]12CC3=CNC4=C3C(=CC=C4)[C@@]1([H])[C@@H](OC(C)=O)[C@H](C)CN2C
Solubility Chemicals:
Soluble in acetone, DMSO or methanol.
Source / Host:
Isolated from Aspergillus sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Nuclear magnetic resonance spectral analysis of the ergot alkaloids: N.J. Bach, et al.; J. Org. Chem. 39, 1272 (1974) | Mycotoxins produced by Aspergillusfumigatus isolated from silage: R.J. Cole, et al.; Ann. Nutr. Aliment. 31, 685 (1977) | Production of fumigaclavine A by Aspergillustamarii Kita: K.K. Janardhanan, et al.; Can. J. Microbiol. 30, 247 (1984) | Abundant respirable ergot alkaloids from the common airborne fungus Aspergillusfumigatus: D.G. Panaccione& C.M. Coyle; Appl. Environ. Microbiol. 71, 3106 (2005) | Post-genome research on the biosynthesis of ergot alkaloids: S.M. Li & I.A. Unsold; Planta Med. 72, 117 (2006) | Reverse prenyltransferase in the biosynthesis of fumigaclavine C in Aspergillus fumigatus: gene expression, purification, and characterization of fumigaclavine C synthase FGAPT1: I.A. Uns?ld & S.M. Li; Chembiochem. 7, 158 (2006) | Clavine Alkaloid biosynthesis by the fungus Penicillium palitans: A.G. Kozlovsky et al.; Appl. Biochem. Microbiol. 45, 182 (2009) | What makes Aspergillus fumigates a successful pathogen?: A. Abad et al.; Rev. Iberoam. Micol. 27, 155 (2010) | Production and characterization of antibodies against fumigaclavine A: H. Latif et al.; Mycotoxin Res. 25, 155 (2010) | Immunochemical analysis of fumigaclavine mycotoxins in respiratory tissues and in blood serum of birds with confirmed aspergillosis: H. Latif, et al.; Mycotoxin Res. 31, 177 (2015) | Ligand and structure-based approaches for the identification of SIRT1 activators: V.K. Vyas, et al.; Chem. Biol. Interact. 228, 9 (2015) | Mycotoxin Identification and In Silico Toxicity Assessment Prediction in Atlantic Salmon: J. Tolosa, et al.; Mar. Drugs 18, 629 (2020)