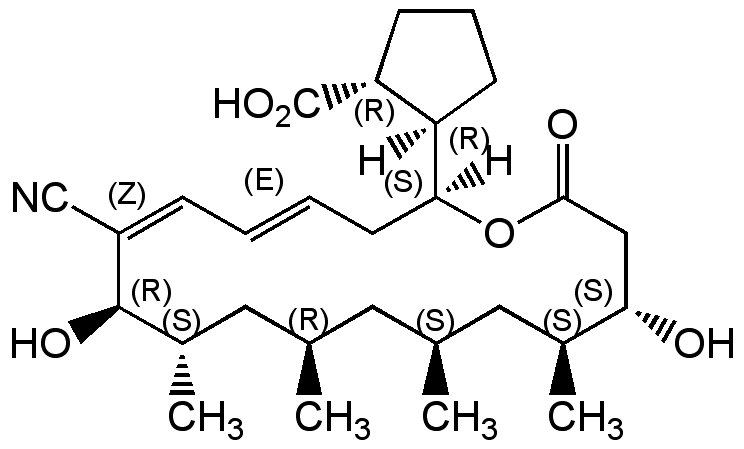
Borrelidin
| Code | Size | Price |
|---|
| BVT-0098-C500 | 500 ug | £95.00 |
Quantity:
| BVT-0098-M001 | 1 mg | £140.00 |
Quantity:
| BVT-0098-M005 | 5 mg | £440.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Treponemycin; U 78548; C2989
Appearance:
White to off-white powder.
CAS:
7184-60-3
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light when in solution.
Hazards:
H302, H312, H319, H332
InChi:
InChI=1S/C28H43NO6/c1-17-12-18(2)14-20(4)27(32)21(16-29)8-5-6-11-25(22-9-7-10-23(22)28(33)34)35-26(31)15-24(30)19(3)13-17/h5-6,8,17-20,22-25,27,30,32H,7,9-15H2,1-4H3,(H,33,34)/b6-5+,21-8-/t17-,18+,19-,20-,22+,23?,24-,25-,27+/m0/s1
InChiKey:
OJCKRNPLOZHAOU-TVHKGLGPSA-N
Long Description:
Chemical. CAS: 7184-60-3. Formula: C28H43NO6. MW: 489.6. Isolated from Streptomyces sp. Macrolide antibiotic. Bacterial, protozoan and mammalian threonyl-tRNA synthetase (THrRS) inhibitor. Antiangiogenic (IC50 = 0.8 nM) and anticancer agent. Induces the collapse of formed capillary tubes in a dose-dependent fashion. In HUVECs, the capillary tube collapsing activity is mediated by the activation of caspases-3 and -8 and induction of apoptosis. Blocks the formation of spontaneous lung metastases of B16-BL6 melanoma cells. Cyclin-dependent kinase (CDK) inhibitor. Antiviral. Potent antimalarial agent (IC50 = 0.97 nM). Inhibitor of potato scab disease.
MDL:
MFCD08276914
Molecular Formula:
C28H43NO6
Molecular Weight:
489.6
Package Type:
Plastic Vial
Precautions:
P261, P270, P280, P301, P312, P302, P352, P312
Product Description:
Macrolide antibiotic. Bacterial, protozoan and mammalian threonyl-tRNA synthetase (THrRS) inhibitor. Antiangiogenic (IC50 = 0.8 nM) and anticancer agent. Induces the collapse of formed capillary tubes in a dose-dependent fashion. In HUVECs, the capillary tube collapsing activity is mediated by the activation of caspases-3 and -8 and induction of apoptosis. Blocks the formation of spontaneous lung metastases of B16-BL6 melanoma cells. Cyclin-dependent kinase (CDK) inhibitor. Antiviral. Potent antimalarial agent (IC50 = 0.97 nM). Inhibitor of potato scab disease.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
[H][C@]1(CCC[C@H]1C(O)=O)[C@]1([H])CC=CC=C(C#N)/[C@H](O)[C@@H](C)C[C@H](C)C[C@H](C)C[C@H](C)[C@@H](O)CC(=O)O1
Solubility Chemicals:
Soluble in DMSO or methanol.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Isolation of vivomycin and borrelidin, two antibiotics with anti-viral activity, from a species of Streptomyces (C2989): M. Lumb, et al.; Nature 206, 263 (1965) | Genetic analysis of mutations causing borrelidin resistance by overproduction of threonyl-transfer ribonucleic acid synthetase: J. Frohler, et al.; J. Bacteriol. 143, 1135 (1980) | Increased levels of threonyl-tRNA synthetase in a borrelidin-resistant Chinese hamster ovary cell line: J.S. Gantt, et al.; PNAS 78, 5367 (1981) | Chinese hamster ovary cells resistant to borrelidin overproduce threonyl-tRNA synthetase: S.C. Gerken and S.M. Arfin; J. Biol. Chem. 259, 9202 (1984) | Isolation of a cDNA clone for human threonyl-tRNA synthetase: amplification of the structural gene in borrelidin-resistant cell lines: K.J. Kontis and S.M. Arfin; Mol. Cell. Biol. 9, 1832 (1989) | Borrelidin is an angiogenesis inhibitor; disruption of angiogenic capillary vessels in a rat aorta matrix culture model: T. Wakabayashi, et al.; J. Antibiot. 50, 671 (1997) | Establishment of a quantitative mouse dorsal air sac model and its application to evaluate a new angiogenesis inhibitor: Y. Funahashi, et al.; Oncol. Res. 11, 319 (1999) | Borrelidin inhibits a cyclin-dependent kinase (CDK), Cdc28/Cln2, of Saccharomyces cerevisiae: E. Tsuchiya, et al.; J. Antibiot. 54, 84 (2001) | Anti-angiogenesis effects of borrelidin are mediated through distinct pathways: threonyl-tRNA synthetase and caspases are independently involved in suppression of proliferation and induction of apoptosis in endothelial cells: T. Kawamura, et al.; J. Antibiot. 56, 709 (2003) | Borrelidin induces the transcription of amino acid biosynthetic enzymes via a GCN4-dependent pathway: E.L. Eastwood & S.E. Schaus; Bioorg. Med. Chem. Lett. 13, 2235 (2003) | A unique hydrophobic cluster near the active site contributes to differences in borrelidin inhibition among threonyl-tRNA synthetases: B. Ruan, et al.; J. Biol. Chem. 280, 571 (2005) | Borrelidin, a potent antimalarial: stage-specific inhibition profile of synchronized cultures of Plasmodium falciparum: A. Ishiyama, et al.; J. Antibiotics 64, 381 (2011) | A study on the effects of borrelidin on cardiovascular and intestinal smooth muscles: D. V. Bhikshapathi, et al.; Pharm. Lett. 5, 83 (2013) | Insights into the preclinical treatment of blood-stage malaria by the antibiotic borrelidin: I. G. Azcarate, et al.; Br. J. Pharmacol. 169, 645 (2013) | Identification of borrelidin binding site on threonyl-tRNA synthetase: M. Li, et al; Biochem. Biophys. Res. Comm. 451, 485 (2014) | Analogs of natural aminoacyl-tRNA synthetase inhibitors clear malaria in vivo: E. M. Novoa, et al.; PNAS (USA) 111, E5508 (2014) | Borrelidin has limited anti-cancer effects in bcl-2 overexpressing breast cancer and leukemia cells and reveals toxicity in non-malignant breast epithelial cells: D. Gafiuc, et al.; J. Appl. Toxicol. 34, 1109 (2014) | Borrelidin or microorganism which produces it as inhibitor of potato scab disease: Y. Kobayashi, et al.; Jap. Kokai Tokkyo Koho JP 2014224102 (2014) | Borrelidin Induces the Unfolded Protein Response in Oral Cancer Cells and Chop-Dependent Apoptosis: A. Sidhu, et al.; ACS Med. Chem. Lett. 6, 1122 (2015) | Effect of borrelidin on hepatocellular carcinoma cells in vitro and in vivo: X. Gao, et al.; RSC Adv. 7, 44401 (2017) | Liposomal borrelidin for treatment of metastatic breast cancer: M. Jeong, et al.; Drug Deliv. Transl. Res. 8, 1380 (2018) | Aminoacyl-tRNA synthetase inhibition activates a pathway that branches from the canonical amino acid response in mammalian cells: Y. Kim, et al.; PNAS 117, 8900 (2020)