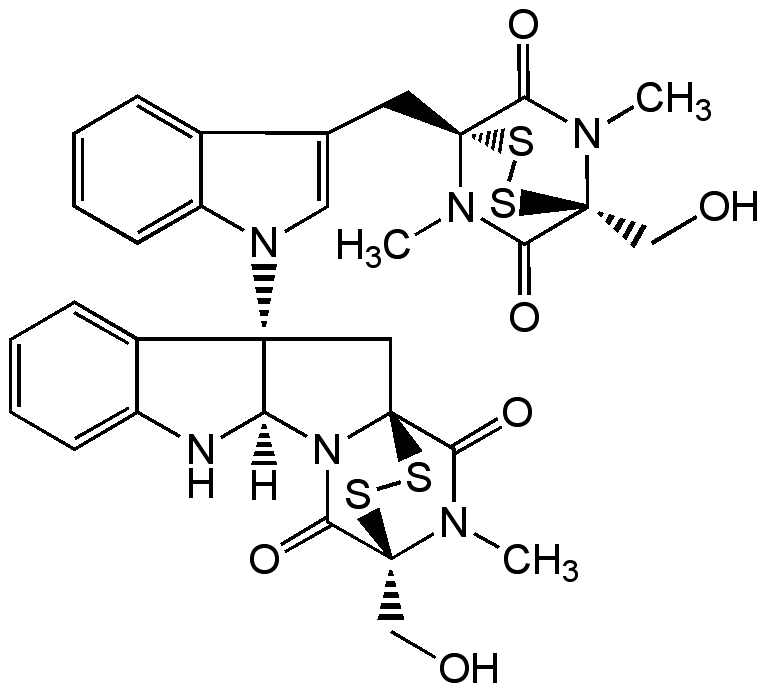
Chetomin
| Code | Size | Price |
|---|
| BVT-0161-M001 | 1 mg | £190.00 |
Quantity:
| BVT-0161-M005 | 5 mg | £640.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
CTM; Chaetomin; NSC289491; BRN0077366
Appearance:
Off-white to fawn solid.
CAS:
1403-36-7
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Protect from moisture.Protect from light when in solution.
Hazards:
H301, H302, H312, H319, H332
InChi:
InChI=1S/C31H30N6O6S4/c1-33-25(42)30(15-38)34(2)23(40)28(33,44-46-30)12-17-13-36(21-11-7-4-8-18(17)21)27-14-29-24(41)35(3)31(16-39,47-45-29)26(43)37(29)22(27)32-20-10-6-5-9-19(20)27/h4-11,13,22,32,38-39H,12,14-16H2,1-3H3/t22-,27+,28-,29+,30+,31+/m1/s1
InChiKey:
ZRZWBWPDBOVIGQ-OKMJTBRXSA-N
Long Description:
Chemical. CAS: 1403-36-7. Formula: C31H30N6O6S4. MW: 710.9. Isolated from Chaetomium sp. Antibiotic. Inhibitor of HIF-1 formation by disrupting the binding of p300 to both HIF-1alpha and HIF-2alpha. Histone H3K9 methyltransferases G9a and Suv39h inhibitor. Tumor growth inhibitor. Angiogenesis inhibitor. Potent immunosuppressor. Antibacterial and antifungal. Strong inducer of retinal pigment epithelium (RPE).
MDL:
MFCD28137713
Molecular Formula:
C31H30N6O6S4
Molecular Weight:
710.9
Package Type:
Plastic Vial
PG:
III
Precautions:
P261, P280, P301, P310, P301, P312, P302, P352, P405
Product Description:
Anticancer antibiotic. Inhibitor of HIF-1 formation by disrupting the binding of p300 to both HIF-1alpha and HIF-2alpha. Histone H3K9 methyltransferases G9a and Suv39h inhibitor. Tumor growth inhibitor. Angiogenesis inhibitor. Potent immunosuppressor. Antibacterial and antifungal. Strong inducer of retinal pigment epithelium (RPE).
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
[H][C@]12NC3=CC=CC=C3[C@]1(C[C@]13SS[C@@](CO)(N(C)C1=O)C(=O)N23)N1C=C(C[C@]23SS[C@@](CO)(N(C)C2=O)C(=O)N3C)C2=CC=CC=C12
Solubility Chemicals:
Soluble in DMSO, ethyl acetate or pyridine; fairly soluble in methanol or 100% ethanol; insoluble in water.
Source / Host:
Isolated from Chaetomium sp.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
The structure of chetomin: A.G. McInnes, et al.; JACS 98, 6741 (1976) | Effects of chetomin on growth and acidic fermentation products of rumen bacteria: W.C. Jen and G.A. Jones; Can. J. Microbiol. 29, 1399 (1983) | Immunomodulatory constituents from an Ascomycete, Chaetomiumseminudum: H. Fujimoto, et al.; J. Nat. Prod. 67, 98 (2004) | Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway: A.L. Kung, et al.; Cancer Cell 6, 33 (2004) | Effects of HIF-1 inhibition by chetomin on hypoxia-related transcription and radiosensitivity in HT 1080 human fibrosarcoma cells: A. Staab, et al.; BMC Cancer 7, 213 (2007) | Epidithiodiketopiperazines block the interaction between hypoxia-inducible factor-1alpha (HIF-1alpha) and p300 by a zinc ejection mechanism: K.M. Cook, et al.; J. Biol. Chem. 284, 26831 (2009) | Direct inhibition of hypoxia-inducible transcription factor complex with designed dimeric epidithiodiketopiperazine: K. M. Wang, et al.; JACS 131, 18078 (2009) | Up-regulation of pro-inflammatory genes as adaptation to hypoxia in MCF-7 cells and in human mammary invasive carcinoma microenvironment: M. Tafani, et al.; Cancer Sci. 101, 1014 (2010) | Inhibition of histone H3K9 methyltransferases by gliotoxin and related epipolythiodioxopiperazines: M. Takahashi, et al. J. Antibiot. 65, 263 (2012) | Epidithiodiketopiperazines (ETPs) exhibit in vitro antiangiogenic and in vivo antitumor activity by disrupting the HIF-1alpha/p300 complex in a preclinical model of prostate cancer: K.M. Reece, et al.; Mol. Cancer 13, 91 (2014) | Small-molecule?directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells: J. Maruotti, et al.; PNAS 112, 10950 (2015) | Chetomin, targeting HIF-1a/p300 complex, exhibits antitumour activity in multiple myeloma: E. Viziteu, et al.; Br. J. Canc. 114, 519 (2016) | Chetomin induces apoptosis in human triple-negative breast cancer cells by promoting calcium overload and mitochondrial dysfunction: J. Dewangan, et al.; BBRC 495, 1915 (2018) | Screening of a growth inhibitor library of sarcoma cell lines to identify potent anti-cancer drugs: Z. Qiao & T. Kondo; Electrophoresis 63, 1 (2019) | Chetomin, a Hsp90/HIF1a pathway inhibitor, effectively targets lung cancer stem cells and non-stem cells: S. Min, et al.; Cancer Biol. Ther. 21, 698 (2020)