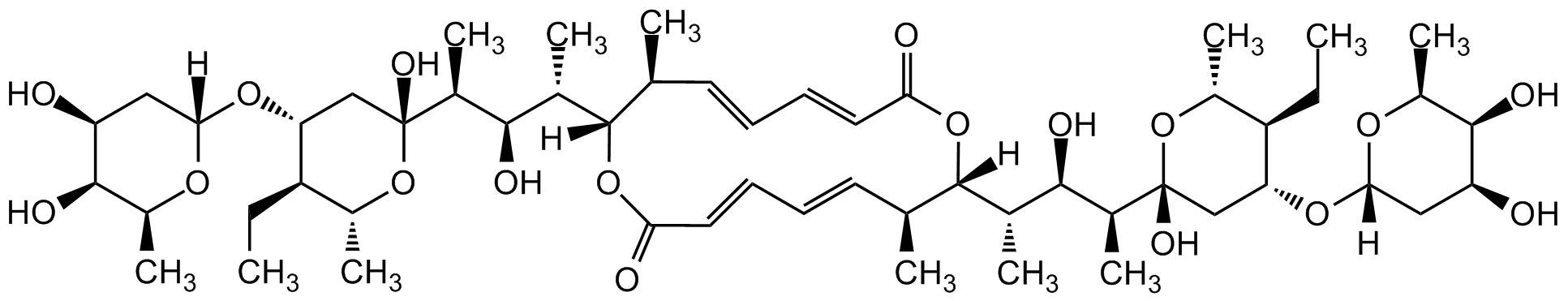
Elaiophylin
| Code | Size | Price |
|---|
| BVT-0185-M001 | 1 mg | £110.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Antibiotic 255-E; Antibiotic 5001B; Antibiotic 56-62; Antibiotic 846I; Gopalamicin; Salbomycin; Azalomycin B; SNA 4606-3
Appearance:
White powder.
CAS:
37318-06-2
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light when in solution.
Hazards:
H302, H319, H332
InChi:
InChI=1S/C54H88O18/c1-13-37-33(9)71-53(63,25-41(37)67-45-23-39(55)49(61)35(11)65-45)31(7)47(59)29(5)51-27(3)19-15-17-22-44(58)70-52(28(4)20-16-18-21-43(57)69-51)30(6)48(60)32(8)54(64)26-42(38(14-2)34(10)72-54)68-46-24-40(56)50(62)36(12)66-46/h15-22,27-42,45-52,55-56,59-64H,13-14,23-26H2,1-12H3/b19-15+,20-16+,21-18+,22-17+/t27-,28-,29-,30-,31-,32-,33+,34+,35-,36-,37+,38+,39-,40-,41+,42+,45-,46-,47+,48+,49+,50+,51-,52-,53+,54+/m0/s1
InChiKey:
OSERMIPXNLXAPD-MJMYBOKFSA-N
Long Description:
Chemical. CAS: 37318-06-2. Formula: C54H88O18. MW: 1025.3. Isolated from Streptomyces sp. MT-1. Antibiotic. Active against Gram-positive bacteria, protozoa and tumors. Testosterone 5alpha-reductase inhibitor. Nitric oxide synthase (NOS) inhibitor. Immunosuppressive. Enhances the antifungal activity of rapamycin.
MDL:
MFCD01939399
Molecular Formula:
C54H88O18
Molecular Weight:
1025.3
Other data:
May undergo transformation to methyl ketal on storage in methanol. We recommend the use of fresh solutions.
Package Type:
Plastic Vial
Precautions:
P261, P270, P280, P301, P312, P304, P340
Product Description:
Antibiotic. Active against Gram-positive bacteria, protozoa and tumors. Antitumor agent. Autophagy inducer and antiangiogenic agent. Testosterone 5alpha-reductase inhibitor. Nitric oxide synthase (NOS) inhibitor. Immunosuppressive. Enhances the antifungal activity of rapamycin.
Purity:
>95% (HPLC)
Signal Word:
Warning
SMILES:
[H][C@@]1(C[C@H](O)[C@H](O)[C@H](C)O1)O[C@@H]1C[C@@](O)(O[C@H](C)[C@H]1CC)[C@@H](C)[C@H](O)[C@H](C)[C@@]1([H])OC(=O)C=CC=C[C@H](C)[C@]([H])(OC(=O)C=CC=C[C@@H]1C)[C@@H](C)[C@@H](O)[C@H](C)[C@@]1(O)C[C@@H](O[C@@]2([H])C[C@H](O)[C@H](O)[C@H](C)O2)[C@H](CC)[C@@H](C)O1
Solubility Chemicals:
Soluble in 100% ethanol, methanol, dimethyl formamide or DMSO.
Source / Host:
Isolated from Streptomyces sp. MT-1.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
Isolation and characterization of Niphithricins A, B and Elaiophylin, antibiotics produced by Streptomyces violaceoniger: H.-P. Fiedler et al.; J Antibiot. 34, 1107 (1981) | Strukturaufkl?rung von Elaiophylin: Spektroskopische Untersuchungen und Abbau: H. Kaiser & W. Keller-Schierlein; Helv. Chim. Acta 64, 407 (1981) | Röntgenstrukturanalyse von Elaiophylin: K. Neupert-Laves & M. Dobler; Helv. Chim. Acta 65, 262 (1982) | Elaiophylin derivatives and their biological activities: P. Hammann et al.; J. Antibiot. 43, 1431 (1990) | The biogenetic origin of the carbon skeleton and the oxygen atoms of Elaiophylin, a symmetric macrodiolide antibiotic: M. Gerlitz et al.; J. Org. Chem. 57, 4030 (1992) | Immunosuppressive activity of elaiophylins: S. Y. Lee et al.; J. Microbiol. Biotechnol. 7, 272 (1997) | SNA-4606-1, a new member of Elaiophylins with enzyme inhibition activity against testosterone 5 alpha-reductase: M. Nakakoshi et al.; J. Antibiot. 52, 175 (1999) | Enhancement of the antifungal activity of Rapamycin by the coproduced Elaiophylin and Nigericin: A. Fang et al.; J. Antibiot. 53, 158 (2000) | Cation selective ion channels formed by macrodiolide antibiotic Elaiophylin in lipid bilayer membranes: P.A. Grigoriev et al.; Bioelectrochem. 54, 11 (2001) | Elaiophylins, new cell cycle inhibitors and apoptosis inducers, produced by Streptomyces pseudoverticillus II. Structures and biological properties: C. Cui et al.; Chinese J. Antibiot. 26, 165-170 (2001) | In vitro and in vivo antiprotozoal activities of bispolides and their derivatives: K. Otoguru et al.; J. Antibiot. 63, 275 (2010) | Elaiophylin, a novel autophagy inhibitor, exerts antitumor activity as a single agent in ovarian cancer cells: X. Zhao, et al.; Autophagy 11, 1849 (2015) | The novel autophagy inhibitor elaiophylin exerts antitumor activity against multiple myeloma with mutant TP53 in part through endoplasmic reticulum stress-induced apoptosis: G. Wang, et al.; Cancer Biol. Ther. 18, 584 (2017) | Antiangiogenic potential of microbial metabolite elaiophylin for targeting tumor angiogenesis: H.N. Lim, et al.; Molecules 23, 563 (2018)