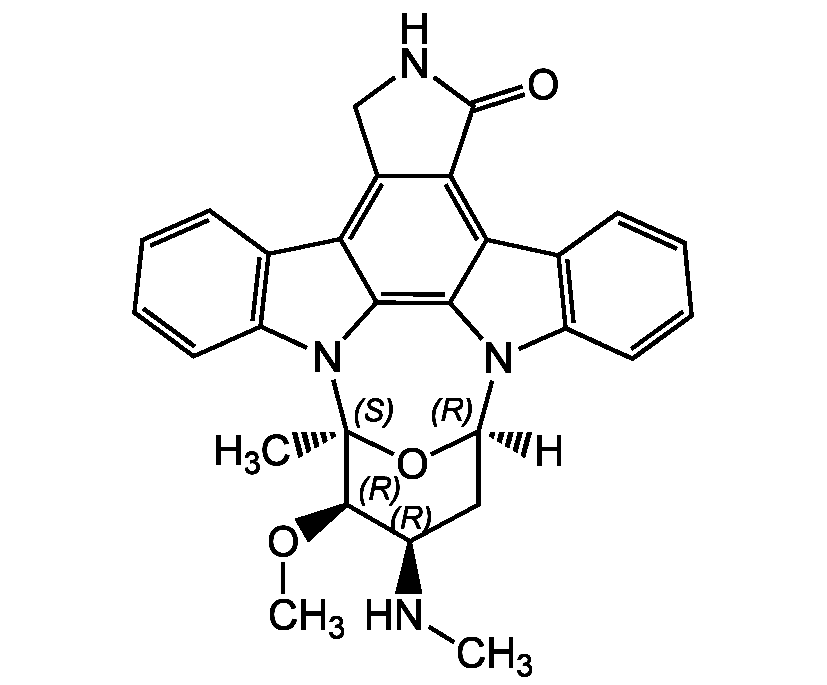
Staurosporine
Product Code: AG-CN2-0022
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0022-C100 | 100 ug | £50.00 |
Quantity:
| AG-CN2-0022-C500 | 500 ug | £95.00 |
Quantity:
| AG-CN2-0022-M001 | 1 mg | £120.00 |
Quantity:
| AG-CN2-0022-M005 | 5 mg | £200.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Antibiotic AM2282; Antibiotic 230; CCRIS 3272
Appearance:
Off-white to yellow solid.
CAS:
62996-74-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Handling Advice:
Keep cool and dry.Protect from moisture.
Hazards:
H340, H350
InChi:
1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1
InChiKey:
HKSZLNNOFSGOKW-FYTWVXJKSA-N
Long Description:
Chemical. CAS: 62996-74-1. Formula: C28H26N4O3. MW: 466.5. Isolated from Streptomyces spiralis. Antibiotic. Antifungal and anti-yeast activity. Inhibits platelet aggregation induced by collagen or ADP. Has no effect on thrombin-induced platelet aggregation. Potent, cell permeable, reversible, ATP-competitive and broad spectrum inhibitor of protein kinases. Inhibits protein kinase A, CaM kinase, myosin light chain kinase, protein kinase C, protein kinase G, CDK1/cyclin B, CDK2/cyclin A, CDK4/cyclin D, CDK5/p25, GSK-3beta and Pim-1 kinase. Key apoptosis inducer. Topoisomerase II (Topo II) inhibitor.
MDL:
MFCD18252446
Molecular Formula:
C28H26N4O3
Molecular Weight:
466.5
Package Type:
Vial
Precautions:
P201, P281, P308, P313, P405
Product Description:
Antibiotic. Antifungal and anti-yeast activity [1]. Inhibits platelet aggregation induced by collagen or ADP. Has no effect on thrombin-induced platelet aggregation [2]. Potent, cell permeable, reversible, ATP-competitive and broad spectrum inhibitor of protein kinases. Inhibits protein kinase A, CaM kinase, myosin light chain kinase, protein kinase C, protein kinase G, CDK1/cyclin B, CDK2/cyclin A, CDK4/cyclin D, CDK5/p25, GSK-3beta and Pim-1 kinase [3, 4, 5, 6, 12]. DYRK1A inhibitor (IC50=20nM) [13]. Key apoptosis inducer [7, 8, 10, 11]. Topoisomerase II (Topo II) inhibitor [9].
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
[H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)N1C3=C(C=CC=C3)C3=C4CNC(=O)C4=C4C5=C(C=CC=C5)N2C4=C13
Solubility Chemicals:
Soluble in DMSO, ethyl acetate or dimethyl formamide. Insoluble in water.
Source / Host:
Isolated from Streptomyces spiralis.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
A new alkaloid AM-2282 of Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization: S. Omura, et al.; J. Antibiot. (Tokyo) 30, 275 (1977) | Staurosporine, a potent platelet aggregation inhibitor from a Streptomyces species: S. Oka, et al.; Agric. Biol. Chem. 50, 2723 (1986) | Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase: T. Tamaoki, et al.; BBRC 135, 397 (1986) | Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60: N. Nakano, et al.; J. Antibiot. (Tokyo) 40, 706 (1987) | Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases: U.T. Ruegg & G.M. Burgess; TIPS 10, 218 (1989) (Review) | Staurosporine: an effective inhibitor for Ca2+/calmodulin-dependent protein kinase II: N. Yanagihara, et al.; J. Neurochem. 56, 294 (1991) | Induction of a common pathway of apoptosis by staurosporine: R. Bertrand, et al.; Exp. Cell Res. 211, 314 (1994) | Staurosporine induces programmed cell death in embryonic neurons and activation of the ceramide pathway: D.A. Wiesner & G. Dawson; J. Neurochem. 66, 1418 (1996) | Mechanism of topoisomerase II inhibition by staurosporine and other protein kinase inhibitors: P. Lassota et al.; J. Biol. Chem. 271, 26418 (1996) | Characterization of the cell death process induced by staurosporine in human neuroblastoma cell lines: J. Boix, et al.; Neuropharmacology 36, 811 (1997) | Molecular mechanism of staurosporine-induced apoptosis in osteoblasts: H.J. Chae, et al.; Pharmacol. Res. 42, 373 (2000) | Pim-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002: M.D. Jacobs, et al.; J. Biol. Chem. 280, 13728 (2005) | Generation of potent and selective kinase inhibitors by combinatorial biosynthesis of glycosylated indolocarbazoles: C. Sanchez, et al.; Chem. Commun. 2009, 4118 (2009)
Related Products
| Product Name | Product Code | Supplier | Purvalanol A | AG-CR1-2903 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AZ191 | AG-CR1-3657 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SB415286 | AG-CR1-3658 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SB216763 | AG-CR1-3659 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||