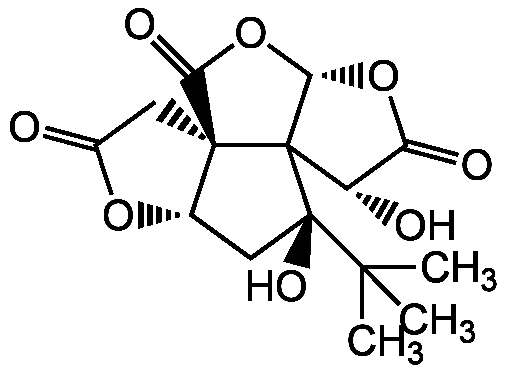
Bilobalide
Product Code: AG-CN2-0026
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0026-M010 | 10 mg | £60.00 |
Quantity:
| AG-CN2-0026-M050 | 50 mg | £210.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Appearance:
White solid.
CAS:
33570-04-6
EClass:
32160000
Form (Short):
liquid
InChi:
InChI=1S/C15H18O8/c1-12(2,3)14(20)4-6-13(5-7(16)21-6)10(19)23-11-15(13,14)8(17)9(18)22-11/h6,8,11,17,20H,4-5H2,1-3H3/t6-,8-,11-,13-,14+,15?/m0/s1
InChiKey:
MOLPUWBMSBJXER-ISSLQHLCSA-N
Long Description:
Chemical. CAS: 33570-04-6. Formula: C15H18O8. MW: 326.3. Isolated from Ginko biloba. Neuroprotective. Mitochondrial gene expression regulator. ROS scavenger. Competitive GABA(A) receptor antagonist. Apoptosis inhibitor. CREB phosphorylation enhancer. Stimulates neurogenesis and synaptogenesis. Hepatic cytochrome P450 inducer. Activates the phosphatidylinositol 3-kinase (PI3K) dependent pathway. Potent anti-inflammatory and antihyperalgesic agent.
MDL:
MFCD00238547
Molecular Formula:
C15H18O8
Molecular Weight:
326.3
Package Type:
Vial
Product Description:
Neuroprotective [2, 3, 4, 5, 7]. Mitochondrial gene expression regulator [3]. ROS scavenger [4]. Competitive GABA(A) receptor antagonist [6]. Apoptosis inhibitor [4, 7, 10, 11]. CREB phosphorylation enhancer. Stimulates neurogenesis and synaptogenesis [8]. Hepatic cytochrome P450 inducer [9]. Activates the phosphatidylinositol 3-kinase (PI3K) dependent pathway [10, 11]. Potent anti-inflammatory and antihyperalgesic agent [12].
Purity:
>95% (1H-NMR)
SMILES:
CC(C)(C)[C@]1(O)C[C@@H]2OC(=O)C[C@@]22C(=O)O[C@@H]3OC(=O)[C@H](O)C123
Solubility Chemicals:
Soluble in ethanol or DMSO.
Source / Host:
Isolated from Ginko biloba.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Working aliquots are stable for up to 3 months when stored at -20°C.
References
Bilobalid A, ein neues Sesquiterpen mit tert.-Butyl-Gruppe aus den Bl?ttern von Ginkgo biloba L.: K. Weinges & W. B?hr; Liebigs Ann. Chem. 724, 214 (1969) | Protection of hypoxia-induced ATP decrease in endothelial cells by ginkgo biloba extract and bilobalide: D. Janssens, et al.; Biochem. Pharmacol. 50, 991 (1995) | Protection of mitochondrial respiration activity by bilobalide: D. Janssens, et al.; Biochem. Pharmacol. 58, 109 (1999) | Reactive oxygen species-induced apoptosis in PC12 cells and protective effect of bilobalide: L.J. Zhou & X.Z. Zhu; J. Pharmacol. Exp. Ther. 293, 982 (2000) | Bilobalide and neuroprotection: F.V. Defeudis; Pharmacol. Res. 46, 565 (2002) (Review) | Bilobalide, a sesquiterpene trilactone from Ginkgo biloba, is an antagonist at recombinant alpha1beta2gamma2L GABA(A) receptors: S.H. Huang, et al.; Eur. J. Pharmacol. 464, 1 (2003) | Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death: K. Chandrasekaran, et al.; Pharmacopsychiatry 36 Suppl 1, S89 (2003) | Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons: F. Tchantchou, et al.; J. Alzheimers Dis. 18, 787 (2009) | Time-dependent induction of hepatic cytochrome P450 enzyme activity and mRNA expression by bilobalide in rats: Y. Taki, et al.; J. Pharmacol. Sci. 109, 459 (2009) | Bilobalide prevents apoptosis through activation of the PI3K/Akt pathway in SH-SY5Y cells: C. Shi, et al.; Apoptosis 15, 715 (2010) | Bilobalide regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway: C. Shi, et al.; Neurochem. Int. 59, 59 (2011) | Bilobalide, a unique constituent of Ginkgo biloba, inhibits inflammatory pain in rats: M. Goldie & S. Dolan; Behav. Pharmacol. 24, 298 (2013)
Related Products
| Product Name | Product Code | Supplier | Thiocolchicoside | AG-CN2-0076 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|