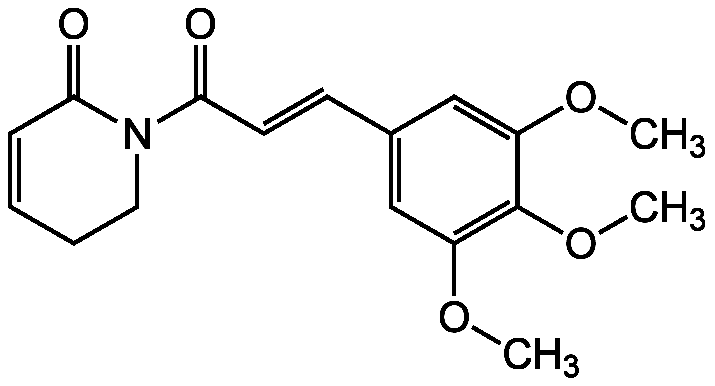
Piperlongumine
Product Code: AG-CN2-0024
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0024-M010 | 10 mg | £40.00 |
Quantity:
| AG-CN2-0024-M050 | 50 mg | £100.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20deg;C
Images
Further Information
Alternate Names/Synonyms:
Piplartine; 5,6-Dihydro-1-(1-oxo-3-[3,4,5-trimethoxyphenyl]-trans-2-propenyl)-2[1H]-pyridinone
Appearance:
White crystals.
CAS:
20069-09-4
EClass:
32160000
Form (Short):
liquid
InChi:
1S/C17H19NO5/c1-21-13-10-12(11-14(22-2)17(13)23-3)7-8-16(20)18-9-5-4-6-15(18)19/h4,6-8,10-11H,5,9H2,1-3H3/b8-7+
InChiKey:
VABYUUZNAVQNPG-BQYQJAHWSA-N
Long Description:
Chemical. CAS: 20069-09-4. Formula: C17H19NO5. MW: 317.3. Isolated from Piper longum roots. Cytotoxic against tumor cell lines. Induces necrosis and apoptosis in cancer cells. Shows anti-platelet aggregation activity possibly by inhibition of cyclooxgenase activity and a decrease in thromboxane A2 formation. Shows significant anxiolytic and antidepressant activities. Promotes adipogenesis of 3T3-L1 cells. Induces in vivo and in vitro mutagenicity in eukaryotic models. Selectively kills cancer cells by targeting the stress response to ROS. Shows in vitro schistosomicidal activity.
MDL:
MFCD00075706
Molecular Formula:
C17H19NO5
Molecular Weight:
317.3
Package Type:
Vial
Product Description:
Cytotoxic against tumor cell lines [3, 4, 5]. Induces necrosis and apoptosis in cancer cells [5, 9, 12]. Shows anti-platelet aggregation activity possibly by inhibition of cyclooxgenase activity and a decrease in thromboxane A2 formation [4, 6, 10]. Shows significant anxiolytic and antidepressant activities [7]. Promotes adipogenesis of 3T3-L1 cells [8]. Induces in vivo and in vitro mutagenicity in eukaryotic models [11]. Selectively kills cancer cells by targeting the stress response to ROS [12]. Shows in vitro schistosomicidal activity [13]. Selective inhibitor of human immunoproteasome. Targets the beta5i subunit (LMP7) (IC50=15µM) with minimal inhibition of human constitutive proteasome [14].
Purity:
>97%
SMILES:
COC1=CC(C=CC(=O)N2CCC=CC2=O)=CC(OC)=C1OC
Solubility Chemicals:
Soluble in DMSO, ethanol, methanol and dimethylformamide.
Source / Host:
Isolated from Piper longum roots.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Alkaloids of Piper longum Linn. I. Structure and synthesis of piperlongumine and piperlonguminine: A. Chatterjee & C.P Dutta; Tetrahedron 23, 1769 (1967) | Cytotoxic pyridone alkaloids from the leaves of Piper aborescens: C.Y. Duh, et al.; J. Nat. Prod. 53, 1575 (1990) | Antiproliferative effects of two amides, piperine and piplartine, from Piper species: D.P. Bezerra, et al.; Z. Naturforsch. C 60, 539 (2005) | New cytotoxic cyclobutanoid amides, a new furanoid lignan and anti-platelet aggregation constituents from Piper arborescens: I.L. Tsai, et al.; Planta Med. 71, 535 (2005) | Piplartine induces inhibition of leukemia cell proliferation triggering both apoptosis and necrosis pathways: D.P. Bezerra, et al.; Toxicol. In Vitro 21, 1 (2007) | Piperlongumine, a constituent of Piper longum L. inhibits rabbit platelet aggregation as a thromboxane A(2) receptor antagonist: M. Iwashita, et al.; Eur. J. Pharmacol. 570, 38 (2007) | Piplartine, an amide alkaloid from Piper tuberculatum, presents anxiolytic and antidepressant effects in mice: F.F. C?cero Bezerra, et al.; Phytomedicine 14, 605 (2007) | Effects of amide constituents from pepper on adipogenesis in 3T3-L1 cells: H. Zhang, et al.; Bioorg. Med. Chem. Lett. 18, 3272 (2008) | Piplartine induces caspase-mediated apoptosis in PC-3 human prostate cancer cells: E.H. Kong, et al.; Oncol. Rep. 20, 785 (2008) | Antiplatelet effects of piplartine, an alkamide isolated from Piper tuberculatum: possible involvement of cyclooxygenase blockade and antioxidant activity: J.B. Fontenele, et al.; J. Pharm. Pharmacol. 61, 511 (2009) | Piplartine induces genotoxicity in eukaryotic but not in prokaryotic model systems: D.P. Bezerra, et al.; Mutat. Res. 677, 8 (2009) | Selective killing of cancer cells by a small molecule targeting the stress response to ROS: L. Raj, et al.; Nature 475, 231 (2011) | Schistosoma mansoni: In vitro schistosomicidal activity of piplartine: J. Moraes, et al.; Exp. Parasitol. 127, 357 (2011) | Piperlongumine and some of its analogs inhibit selectively the human immunoproteasome over the constitutive proteasome: E. Bosc, et al.; BBRC 496, 961 (2018)
Related Products
| Product Name | Product Code | Supplier | Podophyllotoxin | AG-CN2-0049 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|