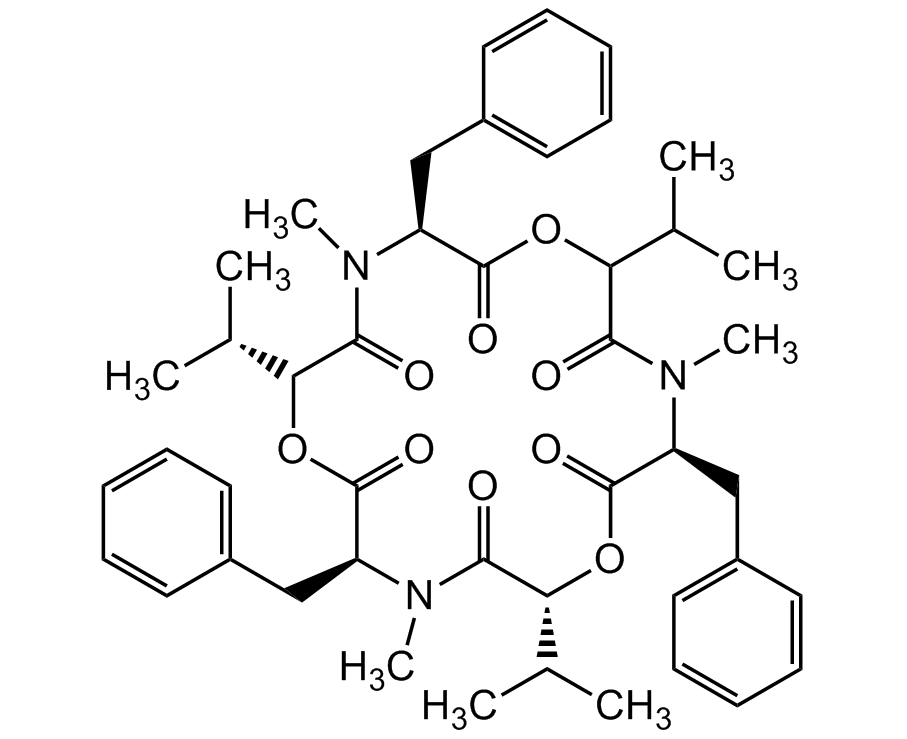
Beauvericin
Product Code: AG-CN2-0043
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0043-M001 | 1 mg | £55.00 |
Quantity:
| AG-CN2-0043-M005 | 5 mg | £150.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Images
Documents
Further Information
Appearance:
White to off-white solid.
CAS:
26048-05-5
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.
InChi:
InChI=1S/C45H57N3O9/c1-28(2)37-40(49)46(7)35(26-32-21-15-11-16-22-32)44(53)56-39(30(5)6)42(51)48(9)36(27-33-23-17-12-18-24-33)45(54)57-38(29(3)4)41(50)47(8)34(43(52)55-37)25-31-19-13-10-14-20-31/h10-24,28-30,34-39H,25-27H2,1-9H3/t34-,35-,36-,37-,38+,39?/m0/s1
InChiKey:
GYSCAQFHASJXRS-GCXUEHFUSA-N
Long Description:
Chemical. CAS: 26048-05-5. Formula: C45H57N3O9. MW: 784. Isolated from fungus Beauveria sp. Antibiotic. Apoptosis inducer. Acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor. Anticancer compound. Antihaptotactic and antimetastatic. Antiangiogenic compound. Antibacterial, antiprotozal, antiviral and antifungal compound. Shows ionophoric properties. Cytotoxic. Genotoxic. Potently interacts with ABCB1 and ABCG2 transport functions. Causes mitochondrial dysfunction.
MDL:
MFCD30541349
Molecular Formula:
C45H57N3O9
Molecular Weight:
784
Package Type:
Vial
Product Description:
Antibiotic [4]. Mycotoxin. Apoptosis inducer [1, 3, 8, 9, 18]. Acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor [2]. Anticancer compound [3, 7, 9]. Antihaptotactic and antimetastatic [10]. Antiangiogenic compound [10]. Antibacterial, antiprotozal, antiviral and antifungal compound [4, 11, 13, 15, 16]. Shows ionophoric properties [5, 17]. Cytotoxic [6, 7]. Genotoxic [14]. Potently interacts with ABCB1 and ABCG2 transport functions [12]. Causes mitochondrial dysfunction [17].
Purity:
>97% (HPLC)
SMILES:
CC.CC(C)C1OC(=O)[C@H](CC2=CC=CC=C2)N(C)C(=O)C(OC(=O)[C@H](CC2=CC=CC=C2)N(C)C(=O)[C@H](OC(=O)[C@H](CC2=CC=CC=C2)N(C)C1=O)C(C)C)C(C)C
Solubility Chemicals:
Soluble in ethanol, methanol or DMSO.
Source / Host:
Isolated from fungus Beauveria sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
Ionophore-induced apoptosis: role of DNA fragmentation and calcium fluxes: D.M. Ojcius, et al.; Exp. Cell Res. 197, 43 (1991) | Inhibition of acyl-CoA: cholesterol acyltransferase activity by cyclodepsipeptide antibiotics: H. Tomoda, et al.; J. Antibiot. (Tokyo) 45, 1626 (1992) | Bcl-2 is overexpressed and alters the threshold for apoptosis in a cholangiocarcinoma cell line: D.M. Harnois, et al.; Hepatology 26, 884 (1997) | Antimycobacterial and antiplasmodial cyclodepsipeptides from the insect pathogenic fungus Paecilomyces tenuipes BCC 1614: C. Nilanonta, et al.; Planta Med. 66, 756 (2000) | Beauvericin-induced channels in ventricular myocytes and liposomes: K. Kouri, et al.; Biochim. Biophys. Acta. 1609, 203 (2003) | Cytotoxic effects of the mycotoxin beauvericin to human cell lines of myeloid origin: L. Cal?, et al.; Pharmacol. Res. 49, 73 (2004) | Beauvericin induces cytotoxic effects in human acute lymphoblastic leukemia cells through cytochrome c release, caspase 3 activation: the causative role of calcium: G.M. Jow, et al.; Cancer Lett. 216, 165 (2004) | Involvement of Bcl-2 family, cytochrome c and caspase 3 in induction of apoptosis by beauvericin in human non-small cell lung cancer cells: H.I. Lin, et al.; Cancer Lett. 230, 248 (2005) | Induction of calcium influx from extracellular fluid by beauvericin in human leukemia cells: B.F. Chen, et al.; BBRC 340, 134 (2006) | Search for cell motility and angiogenesis inhibitors with potential anticancer activity: beauvericin and other constituents of two endophytic strains of Fusarium oxysporum: J. Zhan, et al.; J. Nat. Prod. 70, 227 (2007) | An inhibition study of beauvericin on human and rat cytochrome P450 enzymes and its pharmacokinetics in rats: L. Mei, et al.; J. Enzyme Inhib. Med. Chem. 24, 753 (2009) | Interactions between ABC-transport proteins and the secondary Fusarium metabolites enniatin and beauvericin: R. Dornetshuber, et al.; Mol. Nutr. Food Res. 53, 904 (2009) | Beauvericin and enniatins H, I and MK1688 are new potent inhibitors of human immunodeficiency virus type-1 integrase: C.G. Shin, et al.; J. Antibiot. (Tokyo) 62, 687 (2009) | Beauvericin and ochratoxin A genotoxicity evaluated using the alkaline comet assay: single and combined genotoxic action: M.S. Klari?, et al.; Arch. Toxicol. 84, 641 (2010) | Nematicidal activity of beauvericin produced by the fungus Fusarium bulbicola: A. Shimada, et al.; Z. Naturforsch. C. 65, 207 (2010) | Beauvericin from the endophytic fungus, Fusarium redolens, isolated from Dioscorea zingiberensis and its antibacterial activity: L. Xu, et al.; Nat. Prod. Commun. 5, 811 (2010) | The Fusarium mycotoxins enniatins and beauvericin cause mitochondrial dysfunction by affecting the mitochondrial volume regulation, oxidative phosphorylation and ion homeostasis: A.A. Tonshin, et al.; Toxicology 276, 49 (2010) | Beauvericin induced erythrocyte cell membrane scrambling: S.M. Qadri, et al.; Toxicology 283, 24 (2011)
Related Products
| Product Name | Product Code | Supplier | Rubratoxin A | AG-CN2-0092 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Citrinin | AG-CN2-0101 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||