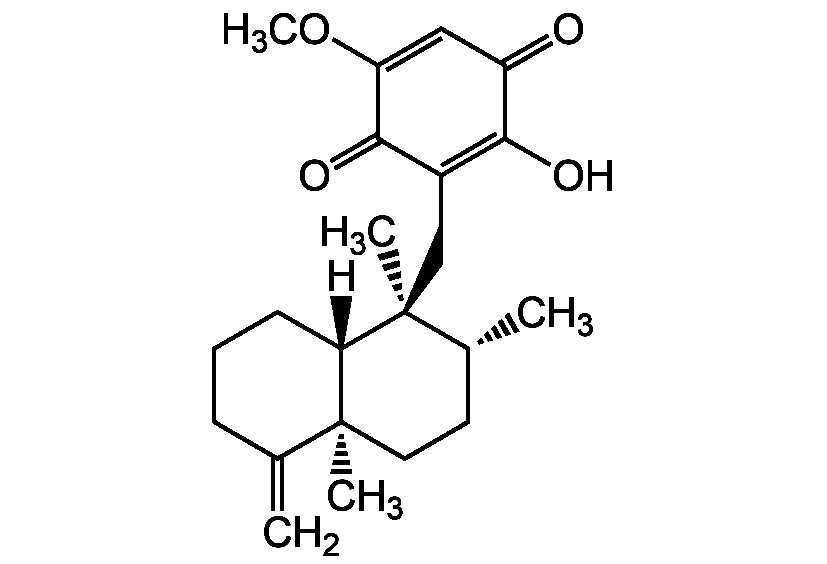
Ilimaquinone
Product Code: AG-CN2-0038
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0038-C100 | 100 ug | £260.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4deg;C
Images
Documents
Further Information
Alternate Names/Synonyms:
3-[(Decahydro-1alpha,2beta,4abeta-trimethyl-5-methylene-1-naphthyl)methyl]-2-hydroxy-5-methoxybenzoquinone; IQ
Appearance:
Yellow solid.
CAS:
71678-03-0
EClass:
32160000
Form (Short):
solid
InChi:
InChI=1S/C22H30O4/c1-13-7-6-8-18-21(13,3)10-9-14(2)22(18,4)12-15-19(24)16(23)11-17(26-5)20(15)25/h11,14,18,24H,1,6-10,12H2,2-5H3/t14-,18+,21+,22+/m1/s1
InChiKey:
JJWITJNSXCXULM-ZKZNVZPYSA-N
Long Description:
Chemical. CAS: 71678-03-0. Formula: C22H30O4. MW: 358.5. Isolated from Anthelia sp. Cytoplasmic microtubule inhibitor. Cell permeable, antimicrobial, anti-inflammatory, antimitotic and cytotoxic compound. Induces a complete and reversible breakdown/disruption of Golgi membranes into smaller vesicular structures. Blocks the association of the ADP-ribosylation factor and beta-COP to the Golgi membrane. Blocks protein transport to the plasma membrane and inhibits gap junctional communication. HIV-1 inhibitor. Blocks the cytotoxicity of ricin and diphtheria toxin. S-Adenosylhomocysteinase hydrolase inhibitor and cellular methylations inhibitor. DNA polymerase beta lyase activity inhibitor. Anti-cancer compound.
MDL:
MFCD22200891
Molecular Formula:
C22H30O4
Molecular Weight:
358.5
Package Type:
Vial
Product Description:
Cytoplasmic microtubule inhibitor [2, 6]. Cell permeable, antimicrobial, anti-inflammatory, antimitotic and cytotoxic compound [1, 11]. Induces a complete and reversible breakdown/disruption of Golgi membranes into smaller vesicular structures [2, 6]. Blocks the association of the ADP-ribosylation factor and beta-COP to the Golgi membrane [3, 6]. Blocks protein transport to the plasma membrane and inhibits gap junctional communication [2, 3, 9]. HIV-1 inhibitor [4]. Blocks the cytotoxicity of ricin and diphtheria toxin [5]. S-Adenosylhomocysteinase hydrolase inhibitor and cellular methylations inhibitor [7, 12]. DNA polymerase beta lyase activity inhibitor [8]. Anti-cancer compound. [10].
Purity:
>98% (HPLC)
SMILES:
[H][C@]12CCCC(=C)[C@]1(C)CC[C@@H](C)[C@]2(C)CC1=C(O)C(=O)C=C(OC)C1=O
Solubility Chemicals:
Soluble in methanol, ethanol, DMSO or hexane.
Source / Host:
Isolated from Anthelia sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Ilimaquinone, a sesquiterpenoid quinone from a marine sponge: R.T. Luibrand, et al.; Tetrahedron 35, 609 (1979) | Microtubule independent vesiculation of Golgi membranes and the reassembly of vesicles into Golgi stacks: B. Veit, et al.; J. Cell. Biol. 122, 1197 (1993) | Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone: P.A. Takizawa, et al.; Cell 73, 1079 (1993) | The interaction of illimaquinone, a selective inhibitor of the RNase H activity, with the reverse transcriptases of human immunodeficiency and murine leukemia retroviruses: S. Loya & A. Hizi; J. Biol. Chem. 268, 9323 (1993) | Ilimaquinone inhibits the cytotoxicities of ricin, diphtheria toxin, and other protein toxins in Vero cells: M.P. Nambiar & H.C. Wu; Exp. Cell Res. 219, 671 (1995) | Golgi-disturbing agents: A. Dinter & E.G. Berger; Histochem. Cell Biol. 109, 571 (1998) | Interactions of (-)-ilimaquinone with methylation enzymes: implications for vesicular-mediated secretion: H.S. Radeke, et al.; Chem. Biol. 6, 639 (1999) | Marine sesquiterpenoids that inhibit the lyase activity of DNA polymerase beta: S. Cao, et al.; J. Nat. Prod. 67, 1716 (2004) | Ilimaquinone inhibits gap junctional communication in a connexin isotype-specific manner: V. Cruciani & S.O. Mikalsen; Exp. Cell Res. 304, 136 (2005) | Ilimaquinone, a marine sponge metabolite, displays anticancer activity via GADD153-mediated pathway: P.H. Lu, et al.; Eur. J. Pharmacol. 556, 45 (2007) | Comparison of the biological properties of several marine sponge-derived sesquiterpenoid quinones: C.A. Motti, et al.; Molecules 12, 1376 (2007) | A new structural class of S-adenosylhomocysteine hydrolase inhibitors: B.G. Kim, et al.; Bioorg. Med. Chem. 17, 6707 (2009)