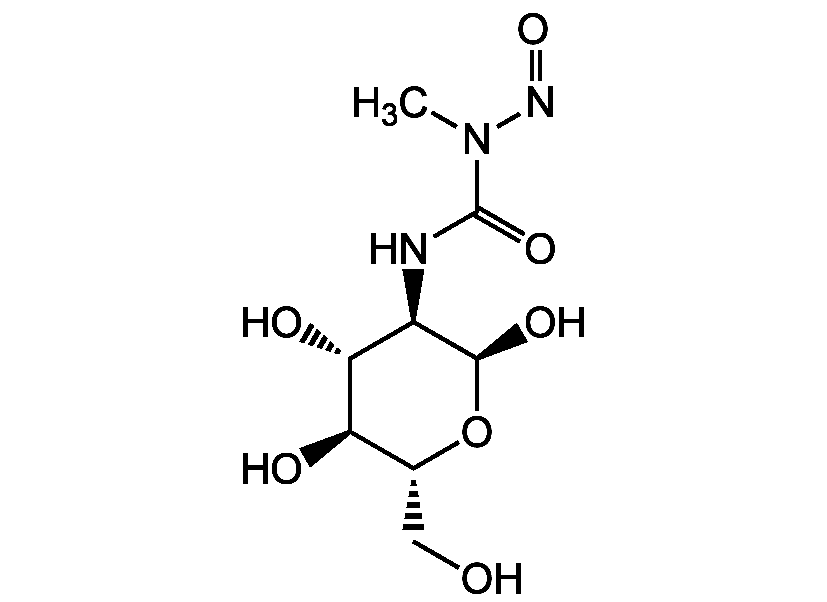
Streptozotocin
Product Code: AG-CN2-0046
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0046-M050 | 50 mg | £35.00 |
Quantity:
| AG-CN2-0046-M250 | 250 mg | £55.00 |
Quantity:
| AG-CN2-0046-G001 | 1 g | £110.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4deg;C
Images
Documents
Further Information
Alternate Names/Synonyms:
2-Deoxy-2-(3-methyl-3-nitrosoureido)-D-glucopyranose; Streptozocin; STZ; NSC 85998; Antibiotic U9889; Zanosar
Appearance:
Off-white to pale yellow powder.
CAS:
18883-66-4
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Handling Advice:
Keep under inert gas.Protect from light.Protect from moisture and oxygen.
Hazards:
H350
InChi:
InChI=1S/C8H15N3O7/c1-11(10-17)8(16)9-4-6(14)5(13)3(2-12)18-7(4)15/h3-7,12-15H,2H2,1H3,(H,9,16)/t3-,4-,5-,6-,7+/m1/s1
InChiKey:
ZSJLQEPLLKMAKR-GKHCUFPYSA-N
Long Description:
Chemical. CAS: 18883-66-4. Formula: C8H15N3O7. MW: 265.2. Antibiotic. Diabetogenic. Diabetes inducer. Induces diabetes mellitus in animal models through its toxic effects on pancreatic beta-cells. Mutagenic. Potent alkylating agent. Potent DNA methylating agent. Nitric oxide (NO) donor. Vasorelaxant. Cytotoxic to cells that express GLUT2 glucose transporter. O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase (O-GlcNAcase) inhibitor. Genotoxic. Induces DNA damage. Produces DNA strand breaks. Cell death inducer. Antineoplastic. Anti-cancer agent used in chemotherapy. Induces cell cylce arrest at G2.
MDL:
MFCD00006607
Molecular Formula:
C8H15N3O7
Molecular Weight:
265.2
Other data:
Note: Once the compound is in solution it spontaneously releases NO gas at room temperature. We recommend to prepare fresh solutions immediately before use.LIT: Anomer-equilibrated streptozotocin solution for the induction of experimental diabetes in mice (Mus musculus): A.S. de la Garza-Rodea, et al.; J. Am. Assoc. Lab. Anim. Sci. 49, 40 (2010)
Package Type:
Vial
Precautions:
P308, P313
Product Description:
Antibiotic [1]. Diabetogenic. Diabetes inducer. Induces diabetes mellitus in animal models through its toxic effects on pancreatic beta-cells [2, 5, 13, 14]. Mutagenic [3, 10]. Potent alkylating agent. Potent DNA methylating agent [4, 10]. Nitric oxide (NO) donor. Vasorelaxant [6]. Cytotoxic to cells that express GLUT2 glucose transporter [7]. O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase (O-GlcNAcase) inhibitor [8]. Genotoxic. Induces DNA damage. Produces DNA strand breaks [9, 10]. Cell death inducer [15]. Antineoplastic. Anti-cancer agent used in chemotherapy [10, 11]. Induces cell cylce arrest at G2 [12].
Purity:
>98% (HPLC) | >75% (alpha anomer)
Signal word:
Danger
SMILES:
CN(N=O)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O
Solubility Chemicals:
Soluble in water or ethanol.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Streptozotocin, a new antibacterial antibiotic: J.J. Vavra, et al.; Antibiot. Ann. 7, 230 (1959) | Studies on the diabetogenic action of Streptozotocin: N. Raketien, et al.; Cancer Chemother. Rep. 29, 91 (1963) | Mutagenic activity of Streptozotocin: S.M. Kolbye & M.S. Legator; Mutat. Res. 6, 387 (1968) | Alkylation of DNA in rat tissues following administration of streptozotocin: R.A. Bennett & A.E. Pegg; Cancer Res. 41, 2786 (1981) | Streptozotocin interactions with pancreatic beta cells and the induction of insulin-dependent diabetes: G.L. Wilson & E.H. Leiter; Curr. Top. Microbiol. Immunol. 156, 27 (1990) (Review) | Nitric oxide generation from streptozotocin: N.S. Kwon, et al.; FASEB J. 8, 529 (1994) | STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells: W.J. Schnedl, et al.; Diabetes 43, 1326 (1994) | Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: linkage of O-linked GlcNAc to beta cell death: K. Liu, et al.; PNAS 97, 2820 (2000) | Chromosomal response of human lymphocytes to Streptozotocin: A.D. Bolzan & M.S. Bianchi; Mutat. Res. 503, 63 (2002) | Genotoxicity of streptozotocin: A.D. Bolzan & M.S. Bianchi; Mutat. Res. 512, 121 (2002) (Review) | Clastogenic effects of streptozotocin on human colon cancer cell lines with gene amplification: A.D. Bolzan & M.S. Bianchi; J. Environ. Pathol. Toxicol. Oncol. 22, 281 (2003) | Streptozotocin induces G2 arrest in skeletal muscle myoblasts and impairs muscle growth in vivo: A.P. Johnston, et al.; Am. J. Physiol. Cell Physiol. 292, C1033 (2007) | The mechanisms of alloxan- and streptozotocin-induced diabetes: S. Lenzen; Diabetologia 51, 216 (2008) | Mechanisms of toxic effect of streptozotocin on beta-cells in the islets of langerhans: V.B. Pisarev, et al.; Bull. Exp. Biol. Med. 148, 937 (2009) | The role of programmed cell death in streptozotocin-induced early diabetic nephropathy: W.H. Wu, et al.; J. Endocrinol. Invest. 34, e296 (2011) | Serelaxin treatment reverses vascular dysfunction and left ventricular hypertrophy in a mouse model of Type 1 diabetes: H.H. Ng, et al.; Sci. Rep. 7, 39604 (2017) | SOCS-1 inhibition of type I interferon restrains Staphylococcus aureus skin host defense: N. Klopfenstein, et al.; PLoS Pathog. 17, e1009387 (2021)
Related Products
| Product Name | Product Code | Supplier | Beauvericin | AG-CN2-0043 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rubratoxin A | AG-CN2-0092 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||