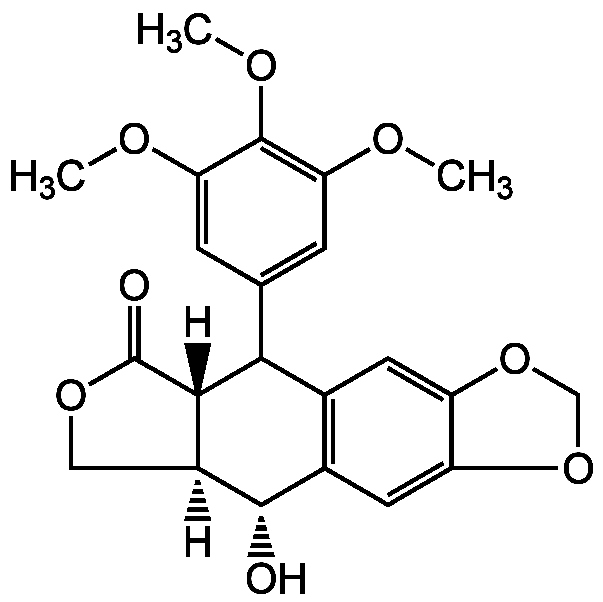
Podophyllotoxin
Product Code: AG-CN2-0049
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0049-M100 | 100 mg | £40.00 |
Quantity:
| AG-CN2-0049-M500 | 500 mg | £100.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4deg;C
Images
Documents
Further Information
Alternate Names/Synonyms:
Podofilox; NSC 24818; Podophyllinic acid lactone
Appearance:
White to off-white powder.
CAS:
518-28-5
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Hygroscopic.Keep under inert gas.Protect from moisture.
Hazards:
H301, H310, H315, H319, H335
InChi:
InChI=1S/C22H22O8/c1-25-16-4-10(5-17(26-2)21(16)27-3)18-11-6-14-15(30-9-29-14)7-12(11)20(23)13-8-28-22(24)19(13)18/h4-7,13,18-20,23H,8-9H2,1-3H3/t13-,18?,19-,20-/m0/s1
InChiKey:
YJGVMLPVUAXIQN-LBXUKJEYSA-N
Long Description:
Chemical. CAS: 518-28-5. Formula: C22H22O8. MW: 414.4. Isolated from Podophyllum emodi rhizomes. Potent microtubule assembly inhibitor. Anticancer compound. Cell death inducer. DNA topoisomerase II inhibitor. Cell cycle inhibitor at the metaphase. Antiviral and antihelminthic.
MDL:
MFCD00075290
Molecular Formula:
C22H22O8
Molecular Weight:
414.4
Package Type:
Vial
PG:
III
Precautions:
P301, P310, P302, P352, P304, P340, P330
Product Description:
Potent microtubule assembly inhibitor [1-3]. Anticancer compound [1-5, 7]. Cell death inducer [1-5, 7]. DNA topoisomerase II inhibitor. Cell cycle inhibitor at the metaphase [2, 6, 8]. Antiviral [3, 4, 9] and antihelminthic [1].
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
[H][C@]12COC(=O)[C@]1([H])C(C1=CC(OC)=C(OC)C(OC)=C1)C1=C(C=C3OCOC3=C1)[C@@H]2O
Solubility Chemicals:
Soluble in chloroform, acetone, ethyl acetate or ethanol. Insoluble in water.
Source / Host:
Isolated from Podophyllum emodi rhizomes.
Transportation:
Excepted Quantity
UN Nummer:
UN 2811
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
VP16-213 and podophyllotoxin. A study on the relationship between chemical structure and biological activity: J.D. Loike; Cancer Chemother. Pharmacol. 7, 103 (1982) | Antitumor agents. I. DNA topoisomerase II inhibitory activity and the structural relationship of podophyllotoxin derivatives as antitumor agents: T. Terada, et al.; Chem. Pharmacol. Bull. (Tokyo) 40, 2720 (1992) | Antineoplastic and antiviral activities of podophyllotoxin related lignans: M. Gordaliza, et al.; Arch. Pharmacol. 327, 175 (1994) | Podophyllotoxin: C. Canel, et al.; Phytochemistry 54, 115 (2000) (Review) | Antitumor properties of podophyllotoxin and related compounds: M. Gordaliza, et al.; Curr. Pharm. Des. 6, 1811 (2000) (Review) | Drugs that inhibit tubulin polymerization: the particular case of podophyllotoxin and analogues: S. Desbene & S. Giorgi-Renault; Curr. Med. Chem. Anticancer Agents 2, 71 (2002) (Review) | Podophyllotoxin derivatives: current synthetic approaches for new anticancer agents: Y. You; Curr. Pharm. Des. 11, 1695 (2005) (Review) | Camptothecin and podophyllotoxin derivatives: inhibitors of topoisomerase I and II - mechanisms of action, pharmacokinetics and toxicity profile: J.T. Hartmann & H.P. Lipp; Drug Saf. 29, 209 (2006) (Review) | An evidence-based review of medical and surgical treatments of genital warts: N. Scheinfeld & D.S. Lehman; Dermatol. Online J. 12, 5 (2006)
Related Products
| Product Name | Product Code | Supplier | Piperlongumine | AG-CN2-0024 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|