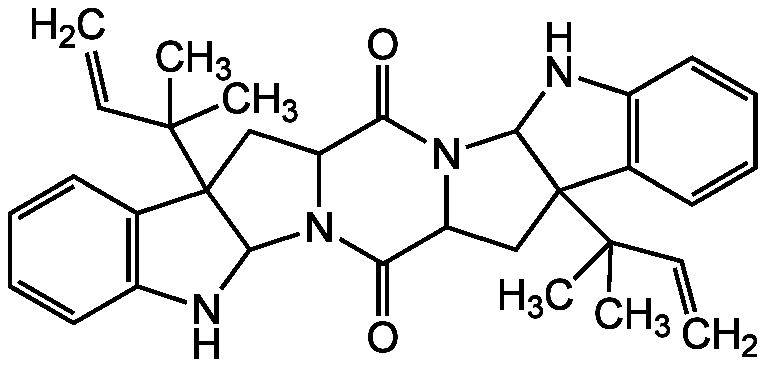
Amauromine
Product Code: AG-CN2-0113
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0113-C250 | 250 ug | £60.00 |
Quantity:
| AG-CN2-0113-M001 | 1 mg | £160.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Antibiotic FR 900220; WF 6237
Appearance:
Brown solid.
CAS:
88360-87-6
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302, H312, H332
InChi:
InChI=1S/C32H36N4O2/c1-7-29(3,4)31-17-23-25(37)36-24(26(38)35(23)27(31)33-21-15-11-9-13-19(21)31)18-32(30(5,6)8-2)20-14-10-12-16-22(20)34-28(32)36/h7-16,23-24,27-28,33-34H,1-2,17-18H2,3-6H3
InChiKey:
VKEAHNPKYMHYJJ-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 88360-87-6. Formula: C32H36N4O2. MW: 508.7. Isolated from Gymnascella sp. Antibiotic. Hypotensive vasodilator. Calcium channel antagonist. Potent and selective CB1 receptor antagonist.
MDL:
MFCD01939909
Molecular Formula:
C32H36N4O2
Molecular Weight:
508.7
Package Type:
Vial
Precautions:
P261, P301, P312, P302, P352, P304, P340
Product Description:
Antibiotic. Hypotensive vasodilator. Calcium channel antagonist. Potent and selective CB1 receptor antagonist.
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
CC(C)(C=C)C12CC3N(C1NC1=C2C=CC=C1)C(=O)C1CC2(C(NC4=C2C=CC=C4)N1C3=O)C(C)(C)C=C
Solubility Chemicals:
Soluble in DMSO, ethanol or methanol.
Source / Host:
Isolated from Gymnascella sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 3 years after receipt when stored at -20°C.
References
Amauromine, a new vasodilator. Taxonomy, isolation and characterization: S. Takase, et al.; J. Antibiot. (Tokyo) 37, 1320 (1984) | Structure of amauromine, a new alkaloid with vasodilating activity produced by sp: S. Takase, et al.; Tetrahedron Lett. 25, 4673 (1984) | Structure of amauromine, a new hypotensive vasodilator produced by sp: S. Takase, et al.; Tetrahedron 41, 3037 (1985) | Total synthesis of amauromine: S. Takase, et al.; Tetrahedron Lett. 26, 847 (1985) | Novoamauromine and ent-Cycloechinulin: two new diketopiperazine derivatives from Aspergillus novofumigatus: K. Ishikawa, et al.; Chem. Pharm. Bull. 58, 717 (2010) | Identification of a Potent and Selective Cannabinoid CB1 Receptor Antagonist from Auxarthron reticulatum: M.F. Elsebai, et al.; ACS Med. Chem. Lett. 2, 866 (2011)