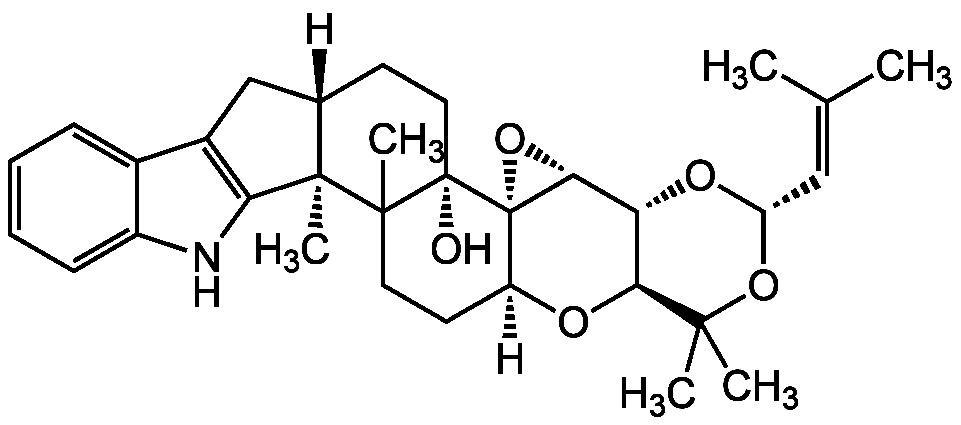
Terpendole C
Product Code: AG-CN2-0125
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0125-C250 | 250 ug | £80.00 |
Quantity:
| AG-CN2-0125-M001 | 1 mg | £220.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Appearance:
Off-white solid.
CAS:
156967-65-6
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302, H312, H332
InChi:
InChI=1/C32H41NO5/c1-17(2)15-23-36-24-26(28(3,4)37-23)35-22-12-13-29(5)30(6)18(11-14-31(29,34)32(22)27(24)38-32)16-20-19-9-7-8-10-21(19)33-25(20)30/h7-10,15,18,22-24,26-27,33-34H,11-14,16H2,1-6H3/t18-,22-,23-,24+,26-,27+,29?,30+,31-,32-/m0/s1
InChiKey:
WUOATFFODCBZBE-SHLFZZOBBH
Long Description:
Chemical. CAS: 156967-65-6. Formula: C32H41NO5. MW: 519.7. Isolated from Albophoma yamanashiensis. Acyl-CoA:cholesterol acyltransferase (ACAT) isozymes ACAT1 and ACAT2 inhibitor. Tremorgenic. Cholesteryl ester (CE) synthesis inhibitor.
Molecular Formula:
C32H41NO5
Molecular Weight:
519.7
Package Type:
Vial
Precautions:
P261, P301, P312, P302, P352, P304, P340
Product Description:
Acyl-CoA:cholesterol acyltransferase (ACAT) isozymes ACAT1 and ACAT2 inhibitor [1-3, 6]. Tremorgenic [4, 5]. Cholesteryl ester (CE) synthesis inhibitor [6, 7].
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
[H][C@]12CC3=C(NC4=CC=CC=C34)[C@]1(C)C1(C)CC[C@]3([H])O[C@H]4[C@@H](O[C@@H](OC4(C)C)C=C(C)C)[C@H]4O[C@@]34[C@]1(O)CC2
Solubility Chemicals:
Soluble in DMSO, ethanol or methanol.
Source / Host:
Isolated from Albophoma yamanashiensis.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 3 years after receipt when stored at -20°C.
References
Microbial metabolites affecting lipid biosynthesis: S. Omura & H. Tomoda; Pure Appl. Chem. 66, 2267 (1994) | Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. I. Production, isolation and biological properties: X.H. Huang, et al.; J. Antibiot. (Tokyo) 48, 1 (1995) | Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. II. Structure elucidation of terpendoles A, B, C and D: X.H. Huang, et al.; J. Antibiot. (Tokyo) 48, 5 (1995) | Isolation and structure elucidation of lolilline, a possible biosynthetic precursor of the lolitrem family of tremorgenic mycotoxins: S.C. Munday-Finch, et al.; J. Agric. Food Chem. 45, 199 (1997) | Terpendole M, a novel indole-diterpenoid isolated from Lolium perenne infected with the endophytic fungus Neotyphodium lolii: W.A. Gatenby, et al.; J. Agric. Food Chem. 47, 1092 (1999) | Selectivity of microbial acyl-CoA:cholesterol acyltransferase inhibitors toward isozymes: T. Ohshiro, et al.; J. Antibiot. (Tokyo) 60, 43 (2007) | Potential therapeutics for obesity and atherosclerosis: Inhibitors of neutral lipid metabolism from microorganisms: H. Tomoda & S. Omura; Pharmacol. Ther. 115, 375 (2007)