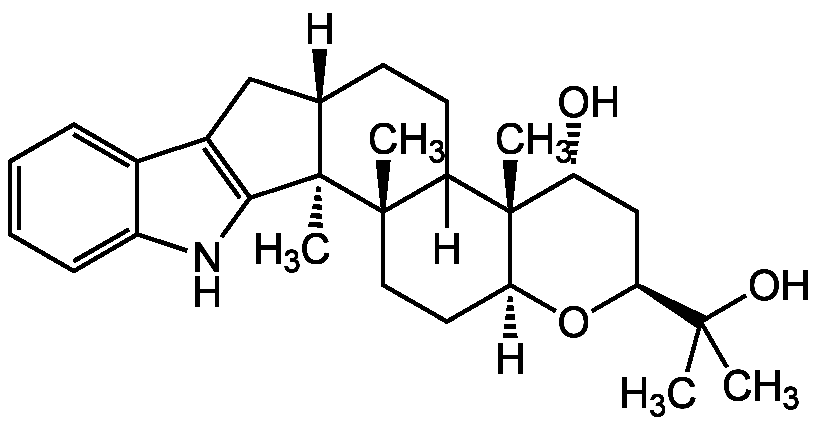
Terpendole E
Product Code: AG-CN2-0127
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0127-C250 | 250 ug | £80.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
1'alpha-Hydroxy-Paspaline
Appearance:
Off-white solid.
CAS:
167427-23-8
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302, H312, H332
InChi:
InChI=1/C28H39NO3/c1-25(2,31)23-15-21(30)27(4)20-11-10-16-14-18-17-8-6-7-9-19(17)29-24(18)28(16,5)26(20,3)13-12-22(27)32-23/h6-9,16,20-23,29-31H,10-15H2,1-5H3/t16-,20?,21+,22-,23-,26-,27+,28+/m0/s1
InChiKey:
SVYIMXYKHRBHSG-CDIJYFRTBB
Long Description:
Chemical. CAS: 167427-23-8. Formula: C28H39NO3. MW: 437.6. Isolated from Albophoma yamanashiensis. Acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor. Mitotic kinesin Eg5 (Mitotic Kinesin Spindle Protein; KSP) inhibitor. Specific M phase inhibitor.
MDL:
MFCD28346948
Molecular Formula:
C28H39NO3
Molecular Weight:
437.6
Package Type:
Vial
Precautions:
P261, P301, P312, P302, P352, P304, P340
Product Description:
Acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor [1, 2]. Mitotic kinesin Eg5 (Mitotic Kinesin Spindle Protein; KSP) inhibitor [3-7]. Specific M phase inhibitor [4].
Purity:
>95% (HPLC)
Signal Word:
Warning
SMILES:
[H][C@]12CC3=C(NC4=CC=CC=C34)[C@]1(C)[C@@]1(C)CC[C@]3([H])O[C@@H](C[C@@H](O)[C@@]3(C)C1([H])CC2)C(C)(C)O
Solubility Chemicals:
Soluble in DMSO, ethanol or methanol.
Source / Host:
Isolated from Albophoma yamanashiensis.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 3 years after receipt when stored at -20°C.
References
Microbial metabolites affecting lipid biosynthesis: S. Omura & H. Tomoda; Pure Appl. Chem. 66, 2267 (1994) | Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. I. Production, isolation and biological properties: X.H. Huang, et al.; J. Antibiot. (Tokyo) 48, 1 (1995) | A novel action of terpendole E on the motor activity of mitotic Kinesin Eg5: J. Nakazawa, et al.; Chem. Biol. 10, 131 (2003) | Development and application of bioprobes for Mammalian cell cycle analyses: H. Osada; Curr. Med. Chem. 10, 727 (2003) (Review) | Docking studies on kinesin spindle protein inhibitors: an important cooperative 'minor binding pocket' which increases the binding affinity significantly: C. Jiang, et al.; J. Mol. Model 13, 987 (2007) | A novel approach to indoloditerpenes by Nazarov photocyclization: synthesis and biological investigations of terpendole E analogues: F. Churruca, et al.; Org. Lett. 12, 2096 (2010) | Kinesin spindle protein (KSP) inhibitors with 2,3-fused indole scaffolds: S. Oishi, et al.; J. Med. Chem. 53, 5054 (2010)