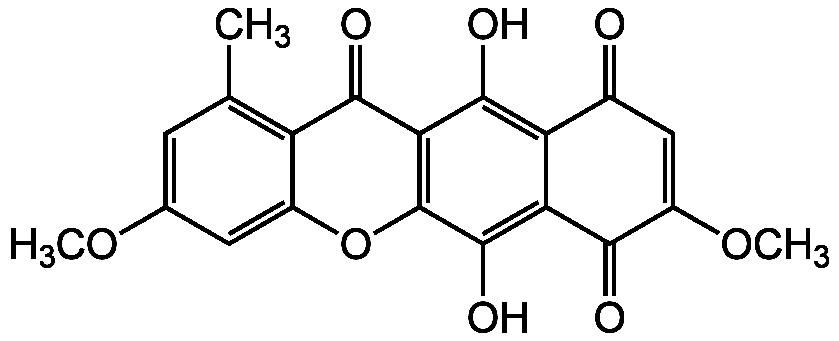
Bikaverin
Product Code: AG-CN2-0130
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0130-C250 | 250 ug | £70.00 |
Quantity:
| AG-CN2-0130-M001 | 1 mg | £190.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NSC 215139; BRN 0358013; Lycopersin; Mycogonin; Passiflorin
Appearance:
Dark red solid.
CAS:
33390-21-5
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302, H312, H332
InChi:
InChI=1S/C20H14O8/c1-7-4-8(26-2)5-10-12(7)17(23)15-18(24)13-9(21)6-11(27-3)16(22)14(13)19(25)20(15)28-10/h4-6,24-25H,1-3H3
InChiKey:
ZOQMSOSJEWBMHP-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 33390-21-5. Formula: C20H14O8. MW: 382.3. Isolated from Fusarium sp. Antibiotic. Antiprotozoal. Antifungal. Anticancer. ATP synthesis inhibitior. Haemolytic agent. Antioomycete. Spermidine-induced autoactivation inhibitor. Plasma hyaluronan-binding protein (PHBP) inhibitor (active form). Review.
MDL:
MFCD01663774
Molecular Formula:
C20H14O8
Molecular Weight:
382.3
Package Type:
Vial
Precautions:
P261, P301, P312, P302, P352, P304, P340
Product Description:
Antibiotic [1]. Antiprotozoal [1]. Antifungal [2]. Anticancer [3-7]. ATP synthesis inhibitior [3, 4]. Haemolytic agent [5]. Antioomycete [8]. Spermidine-induced autoactivation inhibitor [9]. Plasma hyaluronan-binding protein (PHBP) inhibitor (active form) [9]. Review [10].
Purity:
>95% (TLC)
Signal word:
Warning
SMILES:
COC1=CC(C)=C2C(=O)C3=C(OC2=C1)C(O)=C1C(=O)C(OC)=CC(=O)C1=C3O
Solubility Chemicals:
Soluble in DMSO.
Source / Host:
Isolated from Fusarium sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 3 years after receipt when stored at -20°C.
References
Bikaverin, an antibiotic from ibberella fujikoi, effective against Leishmania brasiliensis: J. Balan, et al.; Folia Microbiol. 15, 479 (1970) | Isolation and characterization of a fungal vacuolation factor (bikaverin): J.W. Cornforth, et al.; J. Chem. Soc. Perkin 16, 2786 (1971) | New potential cytotoxic and antitumor substances I. In vitro effect of bikaverin and its derivatives on cells of certain tumors: J. Fuska, et al.; Neoplasma 22, 335 (1975) | Effects of bikaverin on purine nucleotide synthesis and catabolism in Ehrlich ascites tumor cells in vitro: J.F. Henderson, et al.; Biochem. Pharmacol. 26, 1973 (1977) | Inhibition of mitochondrial functions by the antibiotics, bikaverin and duclauxine: L. Kovac, et al.; J. Antibiot. (Tokyo) 31, 616 (1978) | Effects of selected secondary metabolites of Fusarium moniliforme on unscheduled synthesis of DNA by rat primary hepatocytes: W.P. Norred, et al.; Food Chem. Toxicol. 30, 233 (1992) | Search for cell motility and angiogenesis inhibitors with potential anticancer activity: beauvericin and other constituents of two endophytic strains of Fusarium oxysporum: J. Zhan, et al.; J. Nat. Prod. 70, 227 (2007) | Bikaverin and fusaric acid from Fusarium oxysporum show antioomycete activity against Phytophthora infestans: S.W. Son, et al.; J. Appl. Microbiol. 104, 692 (2008) | Purpurin as a specific inhibitor of spermidine-induced autoactivation of the protease plasma hyaluronan-binding protein: N. Nishimura, et al.; Biol. Pharm. Bull. 33, 1430 (2010) | Bikaverin production and applications: M.C. Limon, et al.; Appl. Microbiol. Biotechnol. 87, 21 (2010) (Review)
Related Products
| Product Name | Product Code | Supplier | Aurodox | AG-CN2-0133 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altenusin | AG-CN2-0143 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aspergillimide | AG-CN2-0145 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||