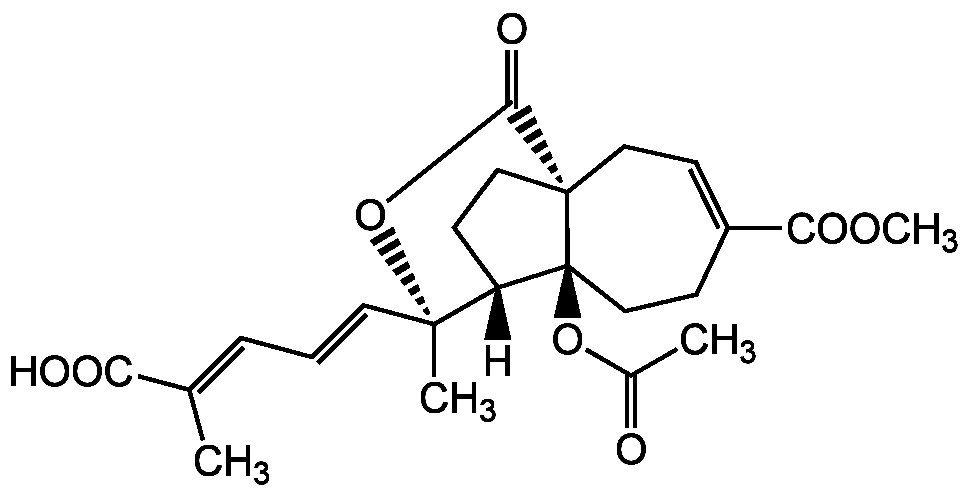
Pseudolaric acid B
Product Code: AG-CN2-0083
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0083-C100 | 100 ug | £35.00 |
Quantity:
| AG-CN2-0083-M001 | 1 mg | £75.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Pseudolarix acid B; PLAB; PAB
Appearance:
White to off-white solid.
CAS:
82508-31-4
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06,GHS08
Handling Advice:
Keep cool and dry.
Hazards:
H301, H361
InChi:
InChI=1S/C23H28O8/c1-14(18(25)26)6-5-10-21(3)17-9-12-22(20(28)31-21)11-7-16(19(27)29-4)8-13-23(17,22)30-15(2)24/h5-7,10,17H,8-9,11-13H2,1-4H3,(H,25,26)/b10-5+,14-6+/t17-,21+,22+,23-/m0/s1
InChiKey:
VDGOFNMYZYBUDT-YDRCMHEVSA-N
Long Description:
Chemical. CAS: 82508-31-4. Formula: C23H28O8. MW: 432.5. Isolated from Pseudolarix kaempferi. Antifungal and antifertility compound. Antitumor compound. PPARalpha signaling agonist. Angiogenesis inhibitor. Apoptosis and autophagy inducer. Microtubule-destabilizing agent. Anti-inflammatory. Inhibits NF-kappaB and p38 signaling. Immunosuppressive.
MDL:
MFCD01746346
Molecular Formula:
C23H28O8
Molecular Weight:
432.5
Package Type:
Vial
PG:
III
Precautions:
P201, P281, P301, P310, P405
Product Description:
Antifungal and antifertility compound [1, 3]. Antitumor compound [2, 7-9, 13]. PPARalpha signaling agonist [4]. Angiogenesis inhibitor [5, 6, 11]. Apoptosis and autophagy inducer [7, 9, 15, 17]. Microtubule-destabilizing agent [8, 11]. Anti-inflammatory. Inhibits NF-kappaB and p38 signaling [12, 14]. Immunosuppressive [16].
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
[H][C@@]12CC[C@]3(CC=C(CC[C@]13OC(C)=O)C(=O)OC)C(=O)O[C@]2(C)C=CC=C(/C)C(O)=O
Solubility Chemicals:
Soluble in DMSO, ethanol, methanol or chloroform.
Source / Host:
Isolated from Pseudolarix kaempferi.
Transportation:
Excepted Quantity
UN Nummer:
UN 2811
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
Pseudolaric Acids from Pseudolarix kaempferi: B.N. Zhou, et al.; Planta Med. 47, 35 (1983) | The cytotoxic principles of Pseudolarix kaempferi: pseudolaric acid-A and -B and related derivatives: D.J. Pan, et al.; Planta Med. 56, 383 (1990) | Antifungal evaluation of pseudolaric acid B, a major constituent of Pseudolarix kaempferi: E. Li, et al.; J. Nat. Prod. 58, 57 (1995) | Pseudolaric acid analogs as a new class of peroxisome proliferator-activated receptor agonists: M.S. Jardat, et al.; Planta Med. 68, 667 (2002) | Pseudolarix acid B inhibits angiogenesis by antagonizing the vascular endothelial growth factor-mediated anti-apoptotic effect: W.F. Tan, et al.; Eur. J. Pharmacol. 499, 219 (2004) | Pseudolaric acid B inhibits angiogenesis and reduces hypoxia-inducible factor 1alpha by promoting proteasome-mediated degradation: M.H. Li, et al.; Clin. Cancer Res. 10, 8266 (2004) | Pseudolaric acid B induces apoptosis through p53 and Bax/Bcl-2 pathways in human melanoma A375-S2 cells: X.F. Gong, et al.; Arch. Pharm. Res. 28, 68 (2005) | Pseudolaric acid B, a novel microtubule-destabilizing agent that circumvents multidrug resistance phenotype and exhibits antitumor activity in vivo: V.K. Wong, et al.; Clin. Cancer Res. 11, 6002 (2005) | Effect of pseudolaric acid B on gastric cancer cells: inhibition of proliferation and induction of apoptosis: K.S. Li, et al.; World J. Gastroenterol. 11, 7555 (2005) | Pseudolarix acid B, a new tubulin-binding agent, inhibits angiogenesis by interacting with a novel binding site on tubulin: Y.G. Tong, et al.; Mol. Pharmacol. 69, 1226 (2006) | Involvement of JNK-initiated p53 accumulation and phosphorylation of p53 in pseudolaric acid B induced cell death: X. Gong, et al.; Exp. Mol. Med. 38, 428 (2006) | Pseudolaric acid B suppresses T lymphocyte activation through inhibition of NF-kappaB signaling pathway and p38 phosphorylation: T. Li, et al.; J. Cell Biochem. 108, 87 (2009) | Selective inhibition of human leukemia cell growth and induction of cell cycle arrest and apoptosis by pseudolaric acid B: G. Ma, et al.; J. Cancer Res. Clin. Oncol. 136, 1333 (2010) | Pseudolaric acid B inhibits inducible cyclooxygenase-2 expression via downregulation of the NF-kappaB pathway in HT-29 cells: L. Hou, et al.; J. Cancer Res. Clin. Oncol. 138, 885 (2012) | Pseudolaric acid B induces apoptosis via proteasome-mediated Bcl-2 degradation in hormone-refractory prostate cancer DU145 cells: D. Zhao, et al.; Toxicol. In Vitro 26, 595 (2012) | The Immunosuppressive Activity of Pseudolaric Acid B on T lymphocytes in vitro: N. Wei, et al: Phytother. Res. 27, 980 (2013) | Pseudolaric Acid B Induces Caspase-Dependent Apoptosis and Autophagic Cell Death in Prostate Cancer Cells: J. Tong, et al.; Phytother. Res. 27, 885 (2013)
Related Products
| Product Name | Product Code | Supplier | Ciglitazone | AG-CR1-0033 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pioglitazone | AG-CR1-0067 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rosiglitazone | AG-CR1-3570 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rosiglitazone . maleate | AG-CR1-3571 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||