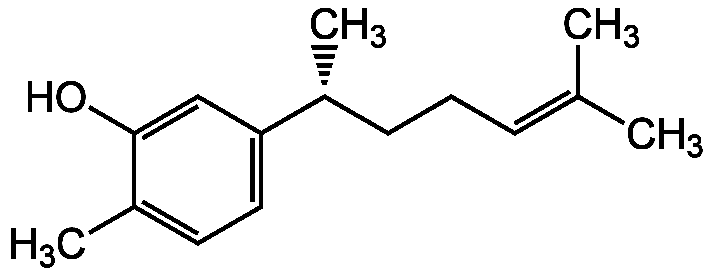
Xanthorrhizol
Product Code: AG-CN2-0090
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0090-M001 | 1 mg | £170.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
1,3,5,10-Bisabolatetraen-2-ol
Appearance:
Colorless to light yellow oil.
CAS:
30199-26-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Handling Advice:
Keep cool and dry.Protect from light.
Hazards:
H360
InChi:
InChI=1S/C15H22O/c1-11(2)6-5-7-12(3)14-9-8-13(4)15(16)10-14/h6,8-10,12,16H,5,7H2,1-4H3/t12-/m1/s1
InChiKey:
FKWGCEDRLNNZOZ-GFCCVEGCSA-N
Long Description:
Chemical. CAS: 30199-26-9. Formula: C15H22O. MW: 218.3. Isolated from Curcuma xanthorrhiza. Shows calcium-antagonistic and vasorelaxant activity. Antibacterial. Anti-inflammatory. Potent COX-2 and inducible nitric oxide (iNOS; NOS II) inhibitor. Hepatoprotective. Anticancer compound. Apoptosis inducer. Neuroprotective antioxidant. Antifungal.
MDL:
MFCD03453037
Molecular Formula:
C15H22O
Molecular Weight:
218.3
Package Type:
Vial
Precautions:
P201, P281, P308, P313, P405
Product Description:
Shows calcium-antagonistic and vasorelaxant activity [1,3]. Antibacterial [2]. Anti-inflammatory. Potent COX-2 and inducible nitric oxide (iNOS; NOS II) inhibitor [4,5,9]. Hepatoprotective [5, 6]. Anticancer compound. Apoptosis inducer [7,8,10,12]. Neuroprotective antioxidant [8]. Antifungal [11].
Purity:
>97% (NMR)
Signal Word:
Danger
SMILES:
C[C@H](CCC=C(C)C)C1=CC=C(C)C(O)=C1
Solubility Chemicals:
Soluble in DMSO or ethanol.
Source / Host:
Isolated from Curcuma xanthorrhiza.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Effect of xanthorrhizol, xanthorrhizol glycoside and trachylobanoic acid isolated from Cachani complex plants upon the contractile activity of uterine smooth muscle: H. Ponce-Monter, et al.; Phytother. Res. 13, 202 (1999) | Antibacterial activity of xanthorrhizol from Curcuma xanthorrhiza against oral pathogens: J.K. Hwang, et al.; Fitoterapia 71, 321 (2000) | Xanthorrhizol induces endothelium-independent relaxation of rat thoracic aorta: M.G. Campos, et al.; Life Sci. 67, 327 (2000) | Suppressive effect of natural sesquiterpenoids on inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) activity in mouse macrophage cells: S.K. Lee, et al.; J. Environ. Pathol. Toxicol. Oncol. 21, 141 (2002) | Abrogation of cisplatin-induced hepatotoxicity in mice by xanthorrhizol is related to its effect on the regulation of gene transcription: S.H. Kim, et al.; Toxicol. Appl. Pharmacol. 196, 346 (2004) | Phosphorylation of c-Jun N-terminal Kinases (JNKs) is involved in the preventive effect of xanthorrhizol on cisplatin-induced hepatotoxicity: K.O. Hong, et al.; Arch. Toxicol. 79, 231 (2005) | Xanthorrhizol induces apoptosis via the up-regulation of bax and p53 in HeLa cells: N. Ismail, et al.; Anticancer Res. 25, 2221 (2005) | Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia: C.S. Lim, et al.; J. Neurosci. Res. 82, 831 (2005) | Regulation of p53-, Bcl-2- and caspase-dependent signaling pathway in xanthorrhizol-induced apoptosis of HepG2 hepatoma cells: T. Handayani, et al.; Anticancer Res. 27, 965 (2007) | Xanthorrhizol inhibits 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation and two-stage mouse skin carcinogenesis by blocking the expression of ornithine decarboxylase, cyclooxygenase-2 and inducible nitric oxide synthase through mitogen-acti: W.Y. Chung, et al.; Carcinogenesis 28, 1224 (2007) | In Vitro antimycotic activity of xanthorrhizol isolated from Curcuma xanthorrhiza Roxb. against opportunistic filamentous fungi: Y. Rukayadi & J.K. Hwang; Phytother. Res. 21, 434 (2007) | Regulation of p53-, Bcl-2- and caspase-dependent signaling pathway in Xanthorrhizol induced DNA fragmentation in HepG2 cells involving Bcl-2 family proteins: BBRC 420, 834 (2012)