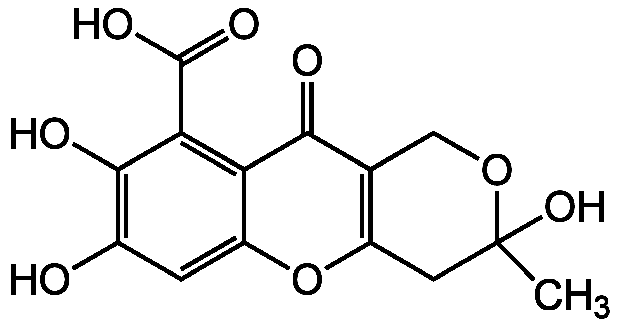
Fulvic acid
Product Code: AG-CN2-0135
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0135-M001 | 1 mg | £130.00 |
Quantity:
| AG-CN2-0135-M005 | 5 mg | £490.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Appearance:
Yellow solid.
CAS:
479-66-3
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302, H312, H332
InChi:
InChI=1S/C14H12O8/c1-14(20)3-8-5(4-21-14)11(16)9-7(22-8)2-6(15)12(17)10(9)13(18)19/h2,15,17,20H,3-4H2,1H3,(H,18,19)
InChiKey:
FCYKAQOGGFGCMD-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 479-66-3. Formula: C14H12O8. MW: 308.2. Isolated from Penicillium sp. strain FKP-0046. Free radical scavenger and antioxidant. Potential compound to neutralize radioactive and toxic wastes and "heal" soils. Chelates and binds scores of minerals into a bioavailable form used by cells. Shown to incorporate into bone and cartilage of rats. Antibacterial. Antifungal. beta-Hexosaminidase release inhibitor. Anti-inflammatory. Tau fibrils aggregation inhibitor (Alzheimers Disease). Antiseptic. Interrupts the dimer formation of Abeta(17-42) peptides.
MDL:
MFCD09838488
Molecular Formula:
C14H12O8
Molecular Weight:
308.2
Package Type:
Vial
Precautions:
P261, P301, P312, P302, P352, P304, P340
Product Description:
Free radical scavenger and antioxidant [1]. Potential compound to neutralize radioactive and toxic wastes and "heal" soils. Chelates and binds scores of minerals into a bioavailable form used by cells [1]. Shown to incorporate into bone and cartilage of rats [2]. Antibacterial [3]. Antifungal [3]. beta-Hexosaminidase release inhibitor [4]. Anti-inflammatory [5]. Tau fibrils aggregation inhibitor (Alzheimers Disease) [6]. Antiseptic [7]. Interrupts the dimer formation of Abeta(17-42) peptides [8].
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
CC1(O)CC2=C(CO1)C(=O)C1=C(C(O)=O)C(O)=C(O)C=C1O2
Solubility Chemicals:
Soluble in methanol, benzene or chloroform. Insoluble in water.
Source / Host:
Isolated from Penicillium sp. strain FKP-0046.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Studies in the biochemistry of micro-organisms: Fulvic acid, a new crystalline yellow pigment, a metabolic product of P. griseo-fulvum Dierckx, P. flexuosum Dale and P. Brefeldianum Dodge: A.E. Oxford, et al.; Biochem. J. 29, 1102 (1935) | The evidence for the incorporation of fulvic acid into the bone and cartilage of rats: C. Wang, et al. Sci. Total Environ. 19, 197 (1996) | An in vitro investigation of the antimicrobial activity of oxifulvic acid: C.E. J. van Rensburg, et al.; J. Antimicrob. Chemother. 46, 853 (2000) | Properties of fulvic acid extracted from excess sludge and its inhibiting effect on beta-hexosaminidase release: H. Motojima, et al.; Biosci. Biotechnol. Biochem. 73, 2210 (2009) | Carbohydrate-derived fulvic acid (CHD-FA) inhibits carrageenan-induced inflammation and enhances wound healing: efficacy and toxicity study in rats: R. Sabi, et al.; Drug Dev. Res. 73, 18 (2011) | Fulvic acid inhibits aggregation and promotes disassembly of tau fibrils associated with Alzheimer's disease: A. Cornejo, et al.; J. Alzheim. Dis. 27, 143 (2011) | Carbohydrate Derived Fulvic Acid: An in vitro Investigation of a Novel Membrane Active Antiseptic Agent Against Candida albicans Biofilms: L. Sherry, et al.; Front. Microbiol. 3, 116 (2012) | The effect of fulvic acid on pre- and postaggregation state of Abeta(17-42): Molecular dynamics simulation studies: S. Verma, et al.; Biochim. Biophys. Acta 1834, 24 (2013)
Related Products
| Product Name | Product Code | Supplier | Shikonin | AG-CN2-0487 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|